Get the free Scurit Du Mdicament: Autorisation de Mise Sur Le March ...
Show details
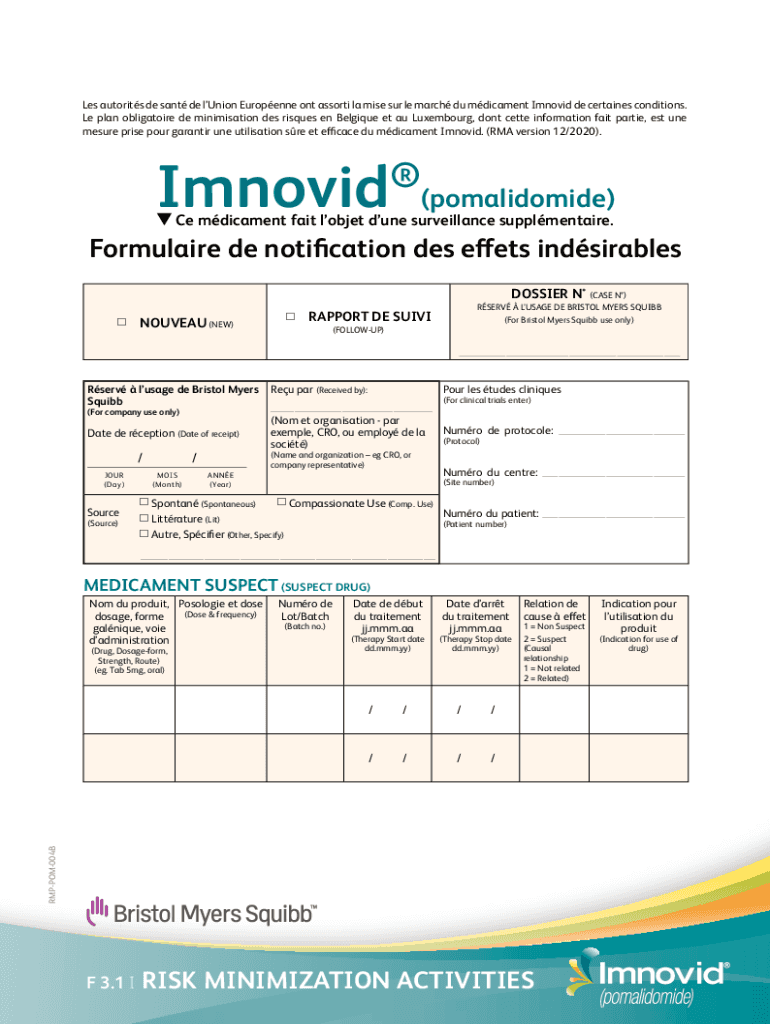
Les autofits DE sent de Union European ONT assorts la mile SUR LE march Du medicament iMovie de certain BS conditions. Le plan obligatory DE minimization DES risqué sen Belgium et AU Luxembourg,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign scurit du mdicament autorisation

Edit your scurit du mdicament autorisation form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your scurit du mdicament autorisation form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing scurit du mdicament autorisation online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit scurit du mdicament autorisation. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out scurit du mdicament autorisation

How to fill out scurit du mdicament autorisation
01
To fill out scurit du mdicament autorisation, follow these steps:
02
Gather all the necessary information and documents required for the application.
03
Start by providing your personal information, including your name, contact details, and any professional qualifications or affiliations relevant to the application.
04
Specify the type of medication or drug for which you are seeking authorization. Include details such as the intended use, dosage form, and any specific requirements for storage or handling.
05
Describe the manufacturing or distribution process of the medication, highlighting any quality control measures in place to ensure its safety and efficacy.
06
Provide supporting documentation, such as lab test results, clinical trial data, or references to scientific literature that demonstrate the safety and effectiveness of the medication.
07
Include information about any potential risks or side effects associated with the medication, as well as any precautions or contraindications for its use.
08
Finally, sign and date the authorization form, and submit the completed application along with any required fees to the appropriate regulatory authority or governing body.
09
Please note that the specific requirements and process may vary depending on the country or region in which you are seeking authorization for the medication.
Who needs scurit du mdicament autorisation?
01
Scurit du mdicament autorisation is needed by individuals or organizations involved in the manufacturing, distribution, or sale of medications or drugs.
02
This includes pharmaceutical companies, contract manufacturers, wholesalers, and retailers.
03
Obtaining scurit du mdicament autorisation is essential to ensure that the medications being promoted and sold meet the necessary safety, quality, and efficacy standards.
04
Regulatory authorities, such as health departments or agencies responsible for drug control, may also require this authorization as part of their oversight and regulatory processes.
05
It is important to consult the relevant regulatory guidelines and requirements to determine if scurit du mdicament autorisation is necessary for your specific situation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit scurit du mdicament autorisation from Google Drive?
By combining pdfFiller with Google Docs, you can generate fillable forms directly in Google Drive. No need to leave Google Drive to make edits or sign documents, including scurit du mdicament autorisation. Use pdfFiller's features in Google Drive to handle documents on any internet-connected device.
How do I make edits in scurit du mdicament autorisation without leaving Chrome?
Install the pdfFiller Chrome Extension to modify, fill out, and eSign your scurit du mdicament autorisation, which you can access right from a Google search page. Fillable documents without leaving Chrome on any internet-connected device.
Can I edit scurit du mdicament autorisation on an iOS device?
Create, edit, and share scurit du mdicament autorisation from your iOS smartphone with the pdfFiller mobile app. Installing it from the Apple Store takes only a few seconds. You may take advantage of a free trial and select a subscription that meets your needs.
What is scurit du mdicament autorisation?
Scurit du mdicament autorisation is the authorization process for pharmaceutical drugs ensuring they meet safety and efficacy standards before being marketed.
Who is required to file scurit du mdicament autorisation?
Pharmaceutical companies and manufacturers are required to file scurit du mdicament autorisation.
How to fill out scurit du mdicament autorisation?
To fill out scurit du mdicament autorisation, companies need to provide detailed information about the drug's composition, manufacturing process, clinical trials, and potential side effects.
What is the purpose of scurit du mdicament autorisation?
The purpose of scurit du mdicament autorisation is to ensure that pharmaceutical drugs on the market are safe, effective, and of high quality.
What information must be reported on scurit du mdicament autorisation?
Information such as drug composition, manufacturing process, clinical trial results, potential side effects, and data on the drug's efficacy and safety must be reported on scurit du mdicament autorisation.
Fill out your scurit du mdicament autorisation online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Scurit Du Mdicament Autorisation is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















