Get the free Class 2 Device Recall Ortho Clinical Diagnostics - Accessdata.fda.gov
Show details
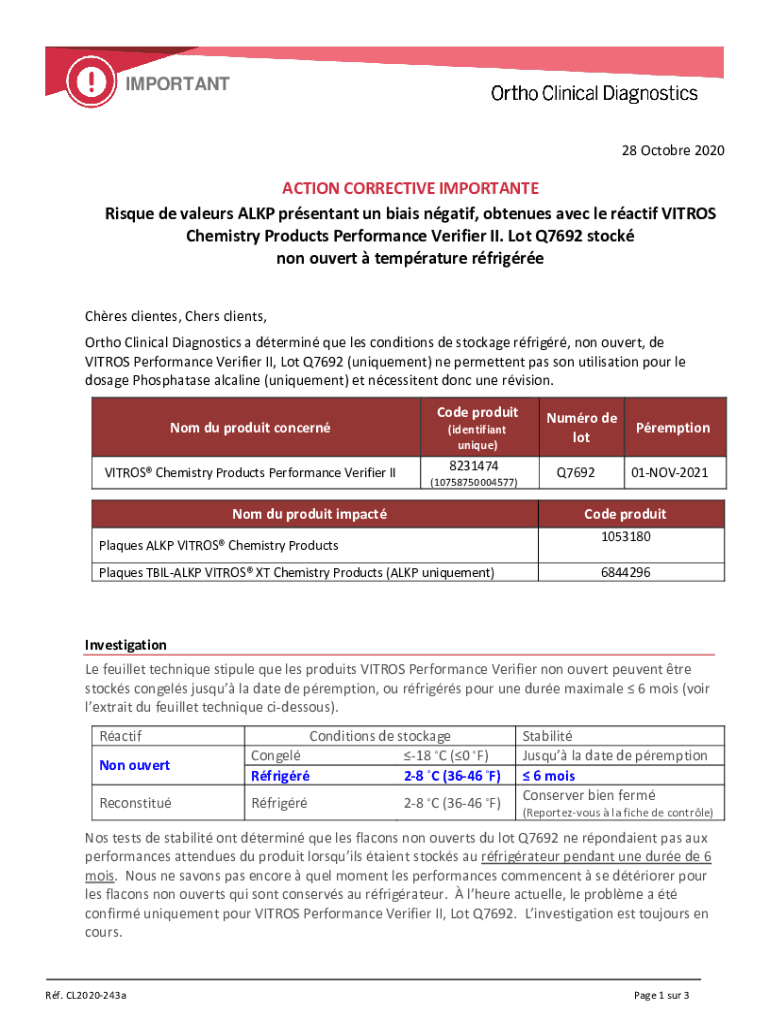
IMPORTANT28 October 2020ACTION CORRECTIVE IMPORTANT Risqué DE valuers ALP pregnant UN bias naif, continues Alec LE ratio VIDEOS Chemistry Products Performance Verifier II. Lot Q7692 stock non-overt
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign class 2 device recall

Edit your class 2 device recall form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your class 2 device recall form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit class 2 device recall online
To use our professional PDF editor, follow these steps:
1
Check your account. In case you're new, it's time to start your free trial.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit class 2 device recall. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out class 2 device recall

How to fill out class 2 device recall
01
Start by gathering all the necessary information about the class 2 device recall, including the product details, reason for the recall, and any specific instructions or forms provided by the regulatory authorities.
02
Identify the affected products and their unique identifiers, such as batch numbers or serial numbers.
03
Inform the relevant stakeholders, including customers, distributors, and healthcare professionals, about the class 2 device recall through appropriate communication channels.
04
Provide clear instructions on how to identify and quarantine the affected products, both within your organization and among the stakeholders.
05
Develop a plan for retrieving the recalled devices, which may involve collection points, return labels, or other logistics arrangements.
06
Implement a method for documenting and tracking the progress of the class 2 device recall, ensuring that all necessary actions are completed.
07
Establish a process for resolving any issues or complaints related to the recall, including providing replacements or refunds if necessary.
08
Keep accurate records of the entire recall process, including communication logs, distribution lists, and any feedback received.
09
Follow up with regulatory authorities as required, providing updates on the progress and completion of the class 2 device recall.
10
Conduct a thorough review and analysis of the recall process, identifying any areas for improvement and implementing corrective actions to prevent similar recalls in the future.
Who needs class 2 device recall?
01
Manufacturers or distributors of class 2 medical devices may need to initiate a class 2 device recall if their products pose a risk to the safety or health of the users. Regulatory authorities may also require a class 2 device recall if they determine that the device does not meet the necessary safety standards or if there have been reported incidents or complaints about the device causing harm or malfunction.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I sign the class 2 device recall electronically in Chrome?
Yes. By adding the solution to your Chrome browser, you may use pdfFiller to eSign documents while also enjoying all of the PDF editor's capabilities in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a photo of your handwritten signature using the extension. Whatever option you select, you'll be able to eSign your class 2 device recall in seconds.
Can I edit class 2 device recall on an iOS device?
Create, modify, and share class 2 device recall using the pdfFiller iOS app. Easy to install from the Apple Store. You may sign up for a free trial and then purchase a membership.
Can I edit class 2 device recall on an Android device?
Yes, you can. With the pdfFiller mobile app for Android, you can edit, sign, and share class 2 device recall on your mobile device from any location; only an internet connection is needed. Get the app and start to streamline your document workflow from anywhere.
What is class 2 device recall?
A class 2 device recall is a notification issued when a medical device may cause temporary or medically reversible adverse health consequences, but the probability of serious harm is remote.
Who is required to file class 2 device recall?
The manufacturer of the medical device, or any entity that has a distribution agreement with the manufacturer, is required to file a class 2 device recall.
How to fill out class 2 device recall?
To fill out a class 2 device recall, you should follow the guidelines provided by the FDA, which typically includes identifying the device, describing the recall reason, providing details on the distribution of the device, and outlining the actions taken to mitigate the issue.
What is the purpose of class 2 device recall?
The purpose of a class 2 device recall is to protect public health by removing or correcting devices that could potentially cause health risks to patients.
What information must be reported on class 2 device recall?
Information that must be reported includes the device name, model and serial numbers, reason for the recall, a description of any adverse events, actions taken by the manufacturer, and a plan for informing affected healthcare providers and patients.
Fill out your class 2 device recall online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Class 2 Device Recall is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















