Get the free Laboratory-identified MDRO or CDI Event for LTCF Doc ...
Show details
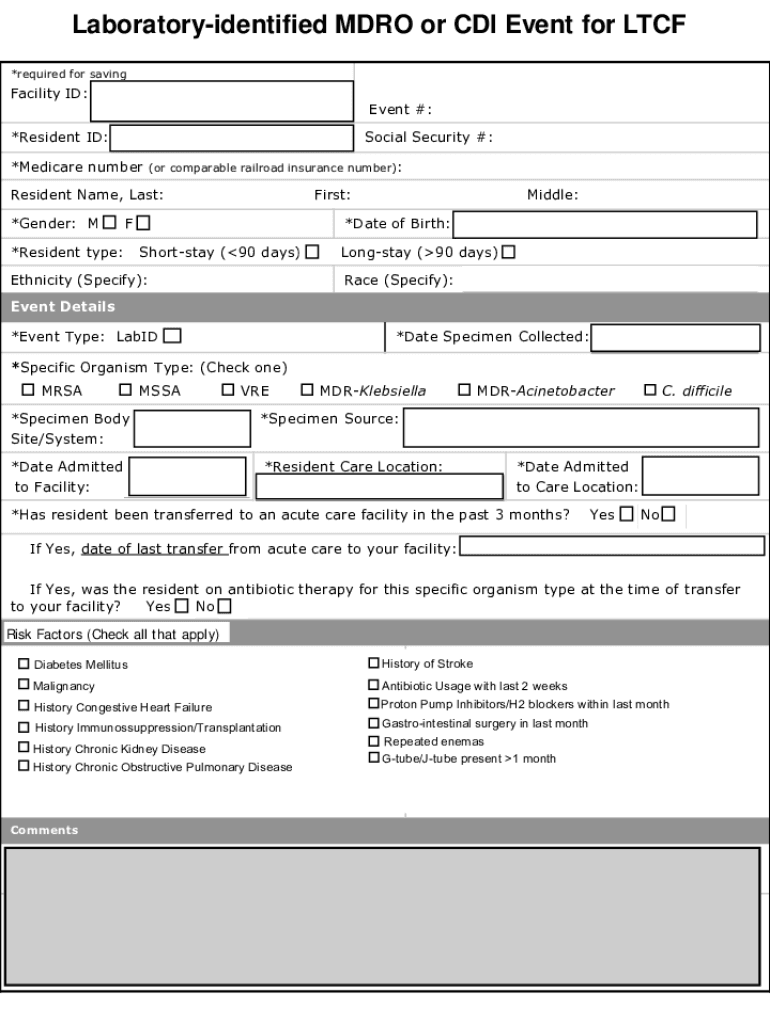
Laboratory identified MDR or CDI Event for LCF *required for savingFacility ID:Event #:*Resident ID:Social Security #:*Medicare number (or comparable railroad insurance number): Resident Name, Last:
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign laboratory-identified mdro or cdi

Edit your laboratory-identified mdro or cdi form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your laboratory-identified mdro or cdi form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit laboratory-identified mdro or cdi online
Use the instructions below to start using our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit laboratory-identified mdro or cdi. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out laboratory-identified mdro or cdi

How to fill out laboratory-identified mdro or cdi
01
Step 1: Gather the necessary materials such as the laboratory-identified mdro or cdi form, pen or pencil, and any additional documentation or instructions provided.
02
Step 2: Start by filling out the patient information section on the form, including their name, date of birth, and medical record number.
03
Step 3: Continue to the test information section and provide details about the specific test being performed, such as the type of specimen collected and the date and time of collection.
04
Step 4: If applicable, indicate any relevant clinical signs or symptoms exhibited by the patient that led to the testing.
05
Step 5: Move on to the laboratory results section and accurately record the findings of the mdro or cdi test, including any relevant measurements or observations.
06
Step 6: Fill in any additional information or comments required by the form, such as the name and contact information of the testing laboratory.
07
Step 7: Review the filled-out form for any errors or omissions, and make sure all required fields have been completed.
08
Step 8: Sign and date the form to certify its accuracy and completion.
09
Step 9: Submit the filled-out laboratory-identified mdro or cdi form to the appropriate personnel or department as per your organization's protocols.
10
Step 10: Keep a copy of the form for your records.
Who needs laboratory-identified mdro or cdi?
01
Laboratory-identified mdro or cdi forms are needed by healthcare organizations, such as hospitals, clinics, and laboratories, that conduct diagnostic testing for multidrug-resistant organisms (mdro) or Clostridium difficile infection (cdi).
02
These forms provide a standardized and documented way to record and report the results of mdro or cdi tests, allowing healthcare professionals to monitor and track the prevalence and spread of these infections.
03
Additionally, public health agencies or regulatory bodies may require healthcare facilities to report mdro or cdi cases, and the laboratory-identified forms serve as the basis for such reporting.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my laboratory-identified mdro or cdi directly from Gmail?
The pdfFiller Gmail add-on lets you create, modify, fill out, and sign laboratory-identified mdro or cdi and other documents directly in your email. Click here to get pdfFiller for Gmail. Eliminate tedious procedures and handle papers and eSignatures easily.
How do I edit laboratory-identified mdro or cdi in Chrome?
Add pdfFiller Google Chrome Extension to your web browser to start editing laboratory-identified mdro or cdi and other documents directly from a Google search page. The service allows you to make changes in your documents when viewing them in Chrome. Create fillable documents and edit existing PDFs from any internet-connected device with pdfFiller.
How do I edit laboratory-identified mdro or cdi straight from my smartphone?
The pdfFiller apps for iOS and Android smartphones are available in the Apple Store and Google Play Store. You may also get the program at https://edit-pdf-ios-android.pdffiller.com/. Open the web app, sign in, and start editing laboratory-identified mdro or cdi.
What is laboratory-identified mdro or cdi?
Laboratory-identified MDRO or CDI refers to Multidrug-Resistant Organisms or Clostridium difficile Infection identified in a laboratory setting.
Who is required to file laboratory-identified mdro or cdi?
Healthcare facilities and laboratories are required to file laboratory-identified MDRO or CDI reports.
How to fill out laboratory-identified mdro or cdi?
Laboratory-identified MDRO or CDI reports can be filled out electronically or using paper forms provided by the relevant health department.
What is the purpose of laboratory-identified mdro or cdi?
The purpose of reporting laboratory-identified MDRO or CDI is to monitor and prevent the spread of antimicrobial resistance and healthcare-associated infections.
What information must be reported on laboratory-identified mdro or cdi?
Information such as patient demographics, specimen source, type of organism, and antimicrobial susceptibility results must be reported on laboratory-identified MDRO or CDI.
Fill out your laboratory-identified mdro or cdi online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Laboratory-Identified Mdro Or Cdi is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















