Get the free www.fda.govclass-ii-special-controls-documentsClass II Special Controls DocumentsFDA
Show details
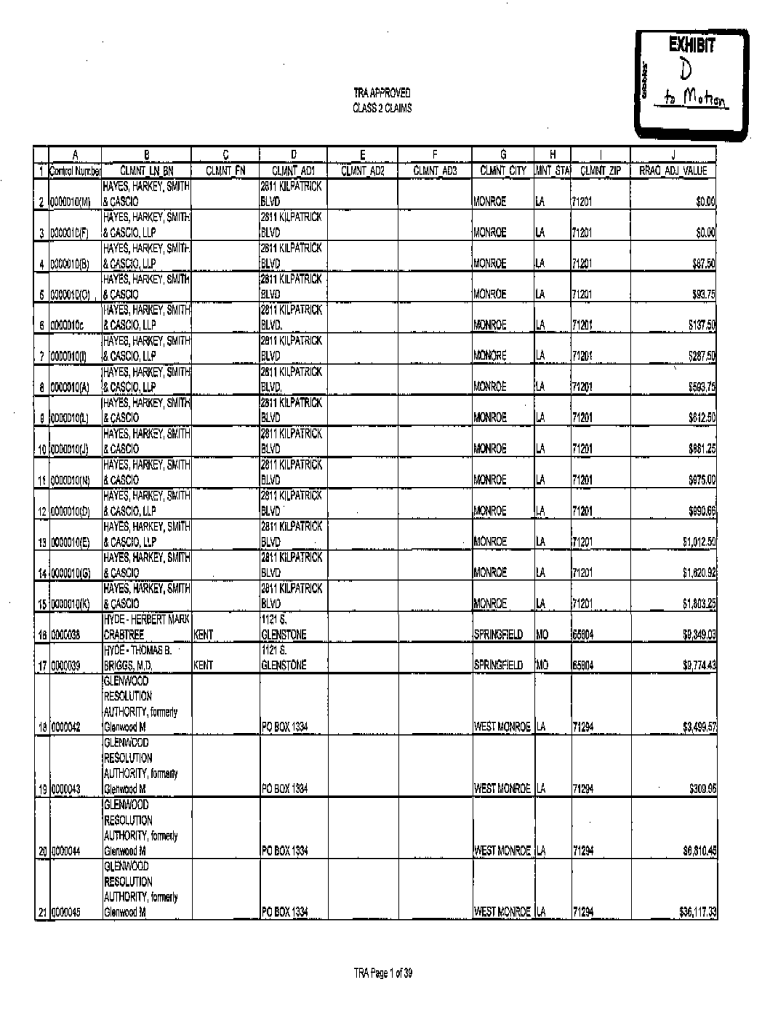
EXHIBITION APPROVED CLASS 2 CLAIMS 1 Control Number 2 0000010(M) 3 0000010(F) 4 0000010(B) 5 0000010(0) 6 0000010c 7 0000010(1) 8 0000010(A) 9 0000010(L) 10 0000010(J) 11 0000010(N) 12 0000010(D)
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign wwwfdagovclass-ii-special-controls-documentsclass ii special controls

Edit your wwwfdagovclass-ii-special-controls-documentsclass ii special controls form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your wwwfdagovclass-ii-special-controls-documentsclass ii special controls form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit wwwfdagovclass-ii-special-controls-documentsclass ii special controls online
To use the services of a skilled PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit wwwfdagovclass-ii-special-controls-documentsclass ii special controls. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out wwwfdagovclass-ii-special-controls-documentsclass ii special controls

How to fill out wwwfdagovclass-ii-special-controls-documentsclass ii special controls
01
To fill out www.fda.gov/class-ii-special-controls-documents/class ii special controls, follow these steps:
02
Open the website www.fda.gov/class-ii-special-controls-documents/class ii special controls in a web browser.
03
Read the instructions provided on the webpage to understand the requirements for filling out the document.
04
Gather all the necessary information and supporting documents that are mentioned in the instructions.
05
Start filling out the form by entering the required information in the designated fields.
06
Double-check your entries to ensure accuracy and completeness.
07
If there are any supporting documents to be attached, follow the specified guidelines to upload them.
08
Review the filled-out document once again to ensure everything is in order.
09
Finally, submit the completed form by following the submission procedure mentioned on the webpage.
10
Keep a copy of the filled-out document and any related confirmation or reference numbers for future reference.
Who needs wwwfdagovclass-ii-special-controls-documentsclass ii special controls?
01
www.fda.gov/class-ii-special-controls-documents/class ii special controls are needed by individuals or companies who are involved in the medical device industry.
02
This may include manufacturers, importers, or distributors of certain class II medical devices.
03
The specific requirements for class II special controls can vary based on the type of medical device and its intended use.
04
Those who wish to market or sell medical devices falling under class II category may require class II special controls documents as part of the regulatory process.
05
It is important to consult the official guidelines provided by the FDA to determine if class II special controls are applicable to your situation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I sign the wwwfdagovclass-ii-special-controls-documentsclass ii special controls electronically in Chrome?
Yes. By adding the solution to your Chrome browser, you can use pdfFiller to eSign documents and enjoy all of the features of the PDF editor in one place. Use the extension to create a legally-binding eSignature by drawing it, typing it, or uploading a picture of your handwritten signature. Whatever you choose, you will be able to eSign your wwwfdagovclass-ii-special-controls-documentsclass ii special controls in seconds.
How do I complete wwwfdagovclass-ii-special-controls-documentsclass ii special controls on an iOS device?
In order to fill out documents on your iOS device, install the pdfFiller app. Create an account or log in to an existing one if you have a subscription to the service. Once the registration process is complete, upload your wwwfdagovclass-ii-special-controls-documentsclass ii special controls. You now can take advantage of pdfFiller's advanced functionalities: adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
Can I edit wwwfdagovclass-ii-special-controls-documentsclass ii special controls on an Android device?
You can make any changes to PDF files, like wwwfdagovclass-ii-special-controls-documentsclass ii special controls, with the help of the pdfFiller Android app. Edit, sign, and send documents right from your phone or tablet. You can use the app to make document management easier wherever you are.
What is wwwfdagovclass-ii-special-controls-documentsclass ii special controls?
Class II special controls are guidelines and requirements set by the FDA to ensure the safety and effectiveness of certain medical devices.
Who is required to file wwwfdagovclass-ii-special-controls-documentsclass ii special controls?
Manufacturers of Class II medical devices are required to comply with the special controls document set by the FDA.
How to fill out wwwfdagovclass-ii-special-controls-documentsclass ii special controls?
Manufacturers need to carefully review the special controls document provided by the FDA and ensure that their medical devices meet all the specified requirements.
What is the purpose of wwwfdagovclass-ii-special-controls-documentsclass ii special controls?
The purpose of Class II special controls is to provide manufacturers with clear guidelines on how to design, produce, and label their medical devices to ensure their safety and effectiveness.
What information must be reported on wwwfdagovclass-ii-special-controls-documentsclass ii special controls?
Manufacturers must report details about the design, materials, performance, and intended use of the medical device as per the special controls document.
Fill out your wwwfdagovclass-ii-special-controls-documentsclass ii special controls online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Wwwfdagovclass-Ii-Special-Controls-Documentsclass Ii Special Controls is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















