Get the free CAPSULE SUMMARY SHEET - Maryland Historical Trust - mht maryland
Show details
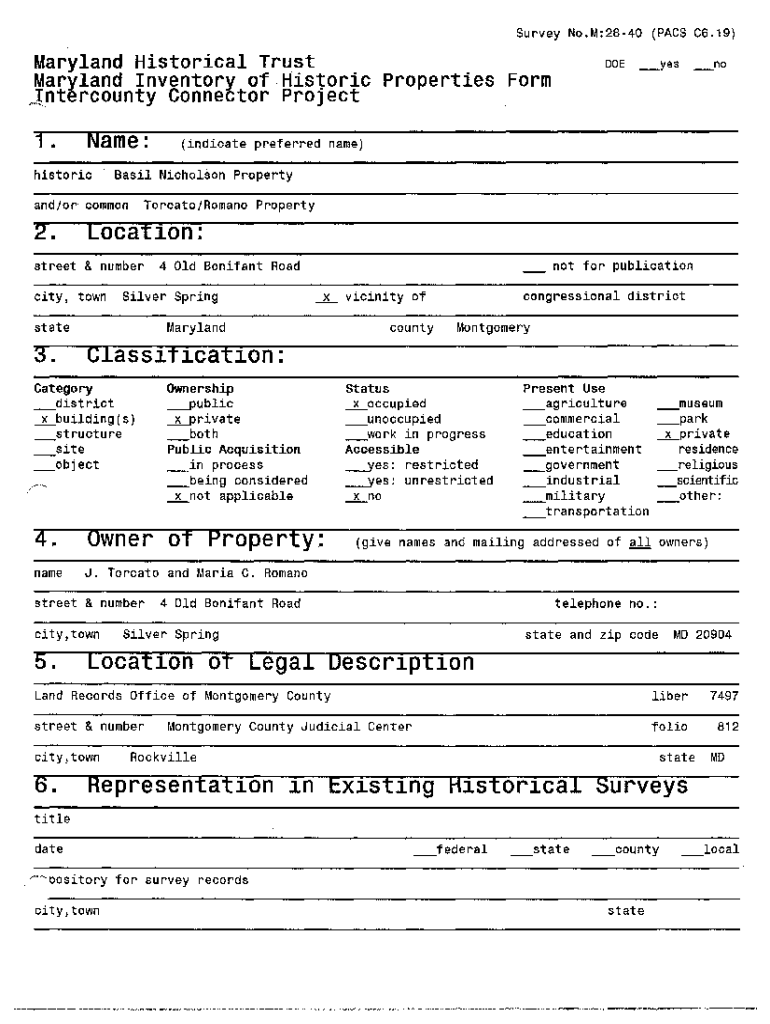
Survey No. M:2840 (PACS C6.19)Maryland Historical Trust Maryland Inventory of. Historic Properties Form.l_ntercounty Connector Project1. Name:historic(indicate preferred name)Location:city, town_4
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign capsule summary sheet

Edit your capsule summary sheet form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your capsule summary sheet form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing capsule summary sheet online
Follow the guidelines below to benefit from a competent PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit capsule summary sheet. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to deal with documents. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out capsule summary sheet

How to fill out capsule summary sheet
01
Start by collecting all the necessary information about the subject or topic that the capsule summary sheet is being filled out for.
02
Write a concise and brief summary of the main points or key elements related to the subject or topic.
03
Organize the summary in a point-by-point format, with each point addressing a specific aspect or idea.
04
Use clear and concise language, avoiding unnecessary jargon or technical terms.
05
Ensure that the summary is accurate and provides a comprehensive overview of the subject or topic.
06
Proofread and revise the summary for clarity and coherence.
Who needs capsule summary sheet?
01
The capsule summary sheet is needed by individuals or organizations involved in research, documentation, or communication of information.
02
It can be useful for journalists, writers, researchers, students, and professionals in various fields.
03
Anyone who needs to quickly understand or convey the main points of a subject or topic can benefit from using a capsule summary sheet.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find capsule summary sheet?
It’s easy with pdfFiller, a comprehensive online solution for professional document management. Access our extensive library of online forms (over 25M fillable forms are available) and locate the capsule summary sheet in a matter of seconds. Open it right away and start customizing it using advanced editing features.
How do I make changes in capsule summary sheet?
The editing procedure is simple with pdfFiller. Open your capsule summary sheet in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I edit capsule summary sheet straight from my smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing capsule summary sheet right away.
What is capsule summary sheet?
A capsule summary sheet is a document that summarizes the essential details and findings of a specific report or study, often used for regulatory or compliance purposes.
Who is required to file capsule summary sheet?
Typically, organizations or individuals involved in regulated activities, such as pharmaceuticals or environmental studies, are required to file a capsule summary sheet.
How to fill out capsule summary sheet?
To fill out a capsule summary sheet, you must provide accurate and concise information including key findings, methodologies, data sources, and conclusions relevant to the summary.
What is the purpose of capsule summary sheet?
The purpose of the capsule summary sheet is to ensure that relevant stakeholders can quickly understand the results and implications of a study or report without reading the entire document.
What information must be reported on capsule summary sheet?
The capsule summary sheet must report the title of the study, authors, date, objectives, key findings, methodologies employed, and any conclusions or recommendations.
Fill out your capsule summary sheet online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Capsule Summary Sheet is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















