Get the free Human Pathogens Importation Regulations R glement sur l ... - brandonu
Show details
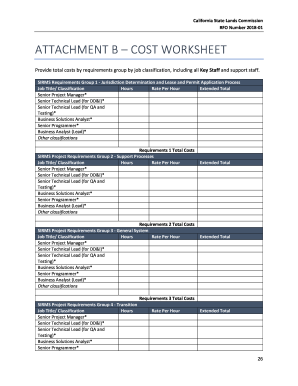
CANADA CONSOLIDATION CODIFICATION Human Pathogens Importation Regulations R element SUR l importation DES agents anthropopathog new FOR/94-558 DOES/94-558 Current to June 2, 2010, four Au 2 join 2010
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign human pathogens importation regulations

Edit your human pathogens importation regulations form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your human pathogens importation regulations form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit human pathogens importation regulations online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit human pathogens importation regulations. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out human pathogens importation regulations

How to fill out human pathogens importation regulations:
01
Familiarize yourself with the regulations: Read through the human pathogens importation regulations to understand the requirements and restrictions in place for importing these materials.
02
Determine if you need to import human pathogens: Assess whether your specific situation requires you to import human pathogens. These regulations typically apply to researchers, laboratories, or medical facilities involved in biological studies or diagnostic testing.
03
Identify the specific pathogens you intend to import: Make a list of the human pathogens that you plan to import. This can include viruses, bacteria, fungi, or other microorganisms.
04
Determine the risk level of the pathogens: Categorize the pathogens based on their risk level. Some pathogens may be classified as high-risk, requiring stricter importation procedures, while others may be low-risk.
05
Obtain the necessary permits and licenses: Contact the relevant authorities or regulatory agencies to find out what permits or licenses are required for importing human pathogens. These may include biosafety permits, import permits, or licenses to handle or store hazardous materials.
06
Complete the required documentation: Fill out all the necessary documentation for importing human pathogens. This may include application forms, importation declarations, or risk assessments. Provide accurate and detailed information about the pathogens, their origin, and intended use.
07
Follow packaging and shipping guidelines: Ensure that the human pathogens are properly packaged and labeled according to the regulations. This may involve using specialized containers, refrigeration, or biohazard labeling.
Who needs human pathogens importation regulations?
01
Researchers: Scientists and researchers involved in studying human pathogens may need to import these materials for laboratory experiments or research purposes. They must comply with the importation regulations to ensure safety and prevent the spread of potentially dangerous pathogens.
02
Laboratories: Diagnostic laboratories that handle and test human samples for infectious diseases may require human pathogens importation regulations. This ensures that they can import and work with specific pathogens needed for accurate diagnostic tests while maintaining biosafety protocols.
03
Medical facilities: Hospitals or medical facilities that conduct clinical trials or research involving human pathogens may need to import these materials. Compliance with importation regulations guarantees the safe and proper handling of pathogens in a medical setting.
Note: It is important to consult the specific regulations and authorities in your country or region to ensure full compliance with the requirements for filling out human pathogens importation regulations.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is human pathogens importation regulations?
Human pathogens importation regulations are guidelines and requirements set by authorities to regulate the importation of microorganisms that can cause disease in humans.
Who is required to file human pathogens importation regulations?
Any individual or organization importing human pathogens for research or other purposes is required to file human pathogens importation regulations.
How to fill out human pathogens importation regulations?
To fill out human pathogens importation regulations, one must provide information about the imported pathogens, the purpose of importation, biosafety measures in place, and other relevant details as specified by the regulations.
What is the purpose of human pathogens importation regulations?
The purpose of human pathogens importation regulations is to prevent the spread of infectious diseases, ensure biosecurity, and protect public health and safety.
What information must be reported on human pathogens importation regulations?
Information that must be reported on human pathogens importation regulations includes details about the imported pathogens (such as species and quantity), the source of the pathogens, the purpose of importation, biosafety measures, and contact information.
How can I manage my human pathogens importation regulations directly from Gmail?
The pdfFiller Gmail add-on lets you create, modify, fill out, and sign human pathogens importation regulations and other documents directly in your email. Click here to get pdfFiller for Gmail. Eliminate tedious procedures and handle papers and eSignatures easily.
How can I modify human pathogens importation regulations without leaving Google Drive?
Using pdfFiller with Google Docs allows you to create, amend, and sign documents straight from your Google Drive. The add-on turns your human pathogens importation regulations into a dynamic fillable form that you can manage and eSign from anywhere.
How do I edit human pathogens importation regulations on an Android device?
You can edit, sign, and distribute human pathogens importation regulations on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
Fill out your human pathogens importation regulations online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Human Pathogens Importation Regulations is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.