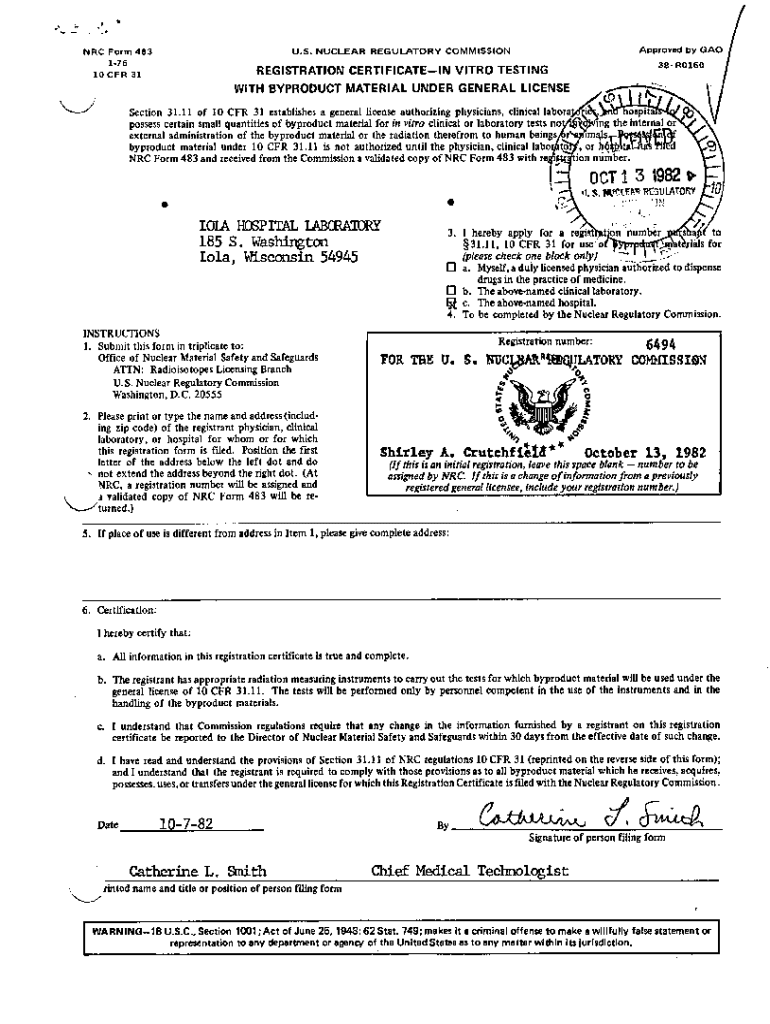
Get the free Registration Certificate for In-Vitro Testing for Iola Hospital, Laboratory.
Show details
J .NRC Form 483 176 10 CFR 31U. S. NUCLEAR REGULATORY COMMISSIONApproved by REGISTRATION CERTIFICATE IN VITO TESTING WITH BYPRODUCT MATERIAL UNDER GENERAL LICENSE38 R0160ISection 31.11 of 10 CFR 31
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign registration certificate for in-vitro

Edit your registration certificate for in-vitro form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your registration certificate for in-vitro form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing registration certificate for in-vitro online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit registration certificate for in-vitro. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out registration certificate for in-vitro

How to fill out registration certificate for in-vitro
01
To fill out a registration certificate for in-vitro, follow these steps:
02
Obtain the registration form from the appropriate regulatory authority.
03
Fill in the required information, such as the name and address of the institution or company applying for the certificate.
04
Provide details about the in-vitro products or devices that require registration, including their intended use and specifications.
05
Attach any supporting documents, such as product labeling, clinical data, or proof of conformity with relevant standards.
06
Pay any applicable fees and submit the completed form along with the supporting documents to the regulatory authority.
07
Wait for the regulatory authority to review the application and issue the registration certificate once all requirements are met.
Who needs registration certificate for in-vitro?
01
Any institution or company involved in the production, distribution, or use of in-vitro diagnostic products or devices needs a registration certificate for in-vitro. This includes manufacturers, importers, exporters, distributors, and healthcare facilities that use in-vitro diagnostics for patient testing and diagnosis.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete registration certificate for in-vitro online?
Easy online registration certificate for in-vitro completion using pdfFiller. Also, it allows you to legally eSign your form and change original PDF material. Create a free account and manage documents online.
How do I edit registration certificate for in-vitro in Chrome?
Get and add pdfFiller Google Chrome Extension to your browser to edit, fill out and eSign your registration certificate for in-vitro, which you can open in the editor directly from a Google search page in just one click. Execute your fillable documents from any internet-connected device without leaving Chrome.
Can I create an eSignature for the registration certificate for in-vitro in Gmail?
You can easily create your eSignature with pdfFiller and then eSign your registration certificate for in-vitro directly from your inbox with the help of pdfFiller’s add-on for Gmail. Please note that you must register for an account in order to save your signatures and signed documents.
What is registration certificate for in-vitro?
The registration certificate for in-vitro is a document that allows companies to legally market and sell in-vitro diagnostic medical devices in a specific country or region.
Who is required to file registration certificate for in-vitro?
Manufacturers and distributors of in-vitro diagnostic medical devices are required to file registration certificates for in-vitro.
How to fill out registration certificate for in-vitro?
To fill out a registration certificate for in-vitro, companies must provide detailed information about the device, its intended use, manufacturing process, and safety and efficacy data.
What is the purpose of registration certificate for in-vitro?
The purpose of the registration certificate for in-vitro is to ensure that in-vitro diagnostic medical devices meet regulatory requirements and are safe and effective for use in diagnosing medical conditions.
What information must be reported on registration certificate for in-vitro?
Information reported on a registration certificate for in-vitro typically includes device details, intended use, manufacturing information, safety and efficacy data, and regulatory compliance.
Fill out your registration certificate for in-vitro online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Registration Certificate For In-Vitro is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















