Get the free Management of Medical Devices Policy - Solent NHS Trust
Show details
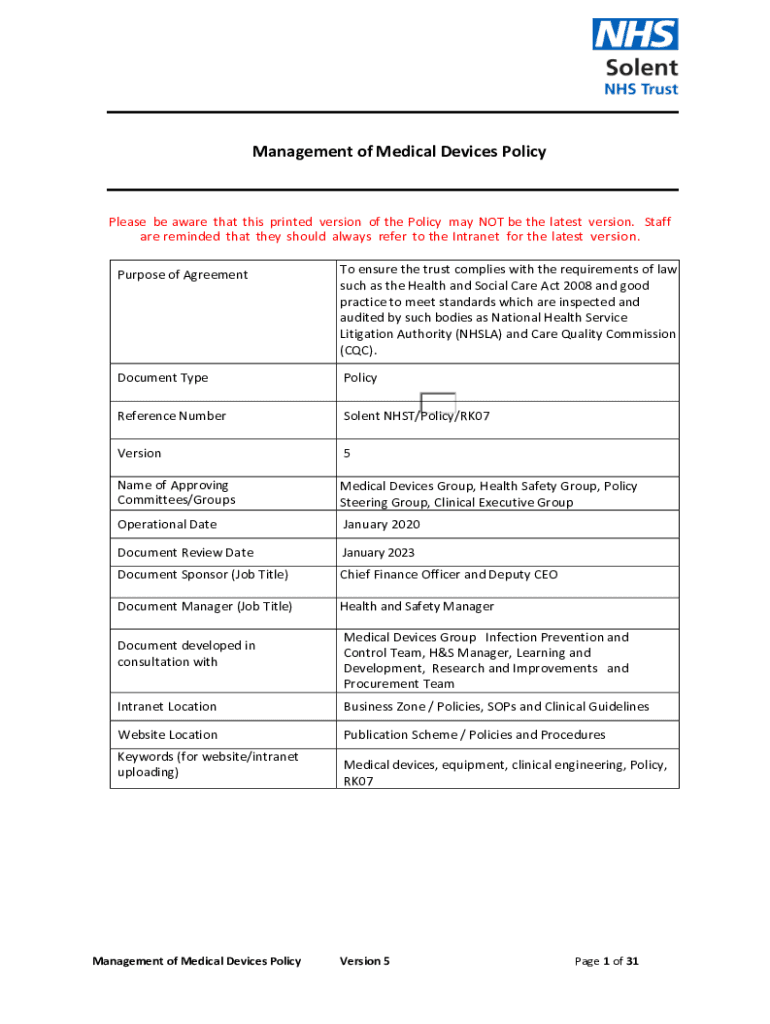
Management of Medical Devices PolicyPlease be aware that this printed version of the Policy may NOT be the latest version. Staff are reminded that they should always refer to the Intranet for the
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign management of medical devices

Edit your management of medical devices form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your management of medical devices form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing management of medical devices online
To use the services of a skilled PDF editor, follow these steps below:
1
Check your account. In case you're new, it's time to start your free trial.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit management of medical devices. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out management of medical devices

How to fill out management of medical devices
01
Start by gathering all the necessary information and documentation related to the medical devices that need management.
02
Review the manufacturer's instructions and guidelines for each device to understand the proper usage, maintenance, and any specific requirements.
03
Create a system for tracking and organizing the medical devices. This can include using software or databases to keep records of device details, such as serial numbers, maintenance history, and expiration dates.
04
Develop a schedule for routine maintenance and inspections of the devices. This may involve coordinating with manufacturers or third-party service providers.
05
Train the relevant staff members on how to use and maintain the medical devices properly. This can include providing hands-on training, conducting workshops, or providing instructional materials.
06
Implement a process for documenting any incidents, malfunctions, or adverse events related to the medical devices. This is important for troubleshooting issues and ensuring patient safety.
07
Stay updated with the latest regulations and guidelines related to medical device management, as they may change over time.
08
Regularly review and evaluate the effectiveness of the medical device management system, making any necessary improvements or adjustments.
09
Maintain accurate records of all activities and documentation related to the management of medical devices for compliance and auditing purposes.
Who needs management of medical devices?
01
Medical facilities such as hospitals, clinics, and healthcare organizations need management of medical devices.
02
Manufacturers and distributors of medical devices also need management systems to ensure quality control, regulatory compliance, and customer satisfaction.
03
Government regulatory bodies and agencies responsible for overseeing healthcare and medical device safety require management of medical devices to enforce regulations and ensure patient safety.
04
Healthcare professionals involved in patient care and treatment rely on the proper management of medical devices to deliver safe and effective healthcare services.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I edit management of medical devices on an iOS device?
You certainly can. You can quickly edit, distribute, and sign management of medical devices on your iOS device with the pdfFiller mobile app. Purchase it from the Apple Store and install it in seconds. The program is free, but in order to purchase a subscription or activate a free trial, you must first establish an account.
How do I complete management of medical devices on an iOS device?
In order to fill out documents on your iOS device, install the pdfFiller app. Create an account or log in to an existing one if you have a subscription to the service. Once the registration process is complete, upload your management of medical devices. You now can take advantage of pdfFiller's advanced functionalities: adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
How do I complete management of medical devices on an Android device?
On Android, use the pdfFiller mobile app to finish your management of medical devices. Adding, editing, deleting text, signing, annotating, and more are all available with the app. All you need is a smartphone and internet.
What is management of medical devices?
Management of medical devices refers to the process of overseeing and controlling the use, distribution, and maintenance of medical devices to ensure they are safe and effective for patients.
Who is required to file management of medical devices?
Manufacturers, importers, and distributors of medical devices are required to file management reports to regulatory authorities.
How to fill out management of medical devices?
Management reports for medical devices typically include information on device specifications, risk assessments, manufacturing processes, quality control measures, and post-market surveillance.
What is the purpose of management of medical devices?
The purpose of management of medical devices is to ensure the safety, effectiveness, and quality of medical devices throughout their lifecycle.
What information must be reported on management of medical devices?
Information that must be reported on management of medical devices includes adverse events, device malfunctions, recalls, and changes in device design or labeling.
Fill out your management of medical devices online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Management Of Medical Devices is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.