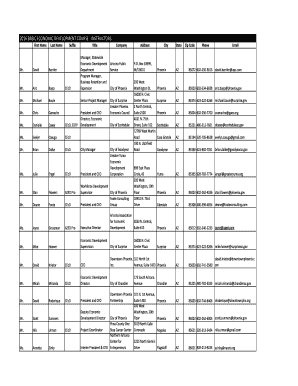
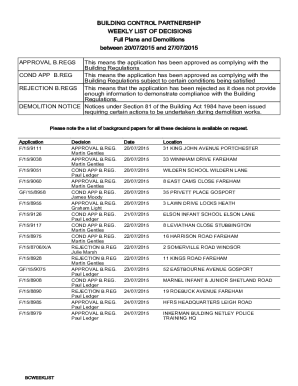
AU SA472 2022 free printable template
Show details
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign centrelink form sa472
Edit your sa472 form download form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your sa472 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing sa472 consent form online
Use the instructions below to start using our professional PDF editor:
1
Log in to your account. Start Free Trial and sign up a profile if you don't have one yet.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit sa472 centrelink form. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
AU SA472 Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out sa472 form centrelink
How to fill out AU SA472
01
Obtain the AU SA472 form from the relevant tax authority website or office.
02
Read the instructions carefully to understand the purpose and requirements of the form.
03
Fill in your personal details including name, address, and tax file number at the top of the form.
04
Answer all relevant questions honestly and thoroughly, ensuring each section is completed.
05
Provide appropriate documentation or references if required by any specific questions.
06
Review the form for accuracy and completeness before submission.
07
Submit the completed AU SA472 form as instructed, either online or by postal mail.
Who needs AU SA472?
01
Individuals or entities seeking to claim a tax deduction or credit in Australia.
02
Taxpayers who need to report specific financial situations or adjustments on their tax returns.
03
People who have received a request from the tax office for additional information regarding their tax obligations.
Fill
centrelink sa472 form
: Try Risk Free






People Also Ask about sa472 medical form
What is a sa472 form?
Use this form to confirm that you consent to your treating health providers disclosing relevant information about your disability or medical conditions to us. Download and complete the Consent to disclose medical information form.
How do you write a consent form?
Considerations in preparing the informed consent document: Elements of consent present. Complete explanations. Lay language. Protection of confidentiality. No unproven claims of effectiveness. Device studies include a statement that the study includes an evaluation of the safety of the test article.
What are the 3 types of consent?
There are three types of patient consent you should know about for legal purposes: oral, written and implied consent. Oral consent: This type of consent comprises any verbal permission a patient gives you to conduct treatment.
What consent form means?
A document with important information about a medical procedure or treatment, a clinical trial, or genetic testing. It also includes information on possible risks and benefits. If a person chooses to take part in the treatment, procedure, trial, or testing, he or she signs the form to give official consent.
What content needs to be included in the informed consent form?
For an ethically valid consent, information provided to a research subject should include, but not be limited to: information about the health condition for which the research is proposed; details of the nature and purpose of the research; the expected duration of the subject's participation; a detailed description of
What kind of document is a consent form?
Consent forms, sometimes called release forms, are legal documents that serve as written permission to send or receive information among participating parties. They often inform them of associated use risks and release the provider from associated claims.
What is the content of a consent form?
The consent form must identify the subject's alternatives to participation in the protocol and should offer a discussion of their relative advantages and disadvantages. It is usually not necessary to provide a full account of the risks and benefits of alternative treatments in the research consent form.
What are five things that need to be included in an informed consent form?
Informed Consent Checklist (1998) A statement that the study involves research. An explanation of the purposes of the research. The expected duration of the subject's participation. A description of the procedures to be followed. Identification of any procedures which are experimental.
What is consent with example?
What is consent? Consent is an agreement between participants to engage in sexual activity. Consent should be clearly and freely communicated. A verbal and affirmative expression of consent can help both you and your partner to understand and respect each other's boundaries.
What are the two types of consent?
There are two types of consent that a patient may give to their medical provider: express consent and implied consent. Express consent is typically done in writing, while implied consent is typically conveyed through a patient's actions or conduct.
Why would you use an information sheet and consent form?
This Participant Information Sheet/Consent Form tells you about the research project. It explains the processes involved with taking part. Knowing what is involved will help you decide if you want to take part in the research.
How do you fill out a consent form?
0:43 2:32 How to Fill VFS Consent Form In India For Your Canada Visa - YouTube YouTube Start of suggested clip End of suggested clip Address put your address telephone number email address signature date signed at city country soMoreAddress put your address telephone number email address signature date signed at city country so let's say you're signing at chennai. So china common india comma india.
What are 3 ways to get consent?
Here are some ways to make sure you have a partner's consent: Always ask for consent before you begin having sex or engaging in a sexual activity. Check in with a partner during sex or a sexual activity. Be attentive to nonverbal cues from partners.
What is the difference between information sheet and consent form?
The Consent Form concisely covers the main points of the Participant Information Sheet phrased as statements with which potential participants can agree or disagree.
What is a consent document?
A document with important information about a medical procedure or treatment, a clinical trial, or genetic testing. It also includes information on possible risks and benefits. If a person chooses to take part in the treatment, procedure, trial, or testing, he or she signs the form to give official consent.
How do you explain a consent form?
A consent form is a document that someone signs to show that they will allow something to happen. Consent forms are used in psychology to insure that a person is aware of what they are agreeing to do and of any risks or costs that may exist.
What is an information sheet?
The information sheet should give a brief summary of the research project and its aims, clearly outlining the entire research process in a language accessible for a non-expert audience. It should also outline what participation means in practice; how long participation takes, where it takes place and what it involves.
How do I fill out a consent form?
0:43 2:32 Address put your address telephone number email address signature date signed at city country soMoreAddress put your address telephone number email address signature date signed at city country so let's say you're signing at chennai. So china common india comma india.
What is an example of written consent?
I have read and I understand the provided information and have had the opportunity to ask questions. I understand that my participation is voluntary and that I am free to withdraw at any time, without giving a reason and without cost.
What is included in a consent form?
The consent form must identify the subject's alternatives to participation in the protocol and should offer a discussion of their relative advantages and disadvantages. It is usually not necessary to provide a full account of the risks and benefits of alternative treatments in the research consent form.
Our user reviews speak for themselves
Read more or give pdfFiller a try to experience the benefits for yourself
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the centrelink sa472 in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your sa472 form pdf.
How do I edit sa472 form pdf download straight from my smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing consent to disclose medical information right away.
Can I edit form sa472 on an Android device?
The pdfFiller app for Android allows you to edit PDF files like australia consent information form. Mobile document editing, signing, and sending. Install the app to ease document management anywhere.
What is AU SA472?
AU SA472 is a form used in Australia for reporting the tax obligations of self-assessment entities. It helps assessors understand the income and deductions of taxpayers.
Who is required to file AU SA472?
Entities that are required to file AU SA472 include self-assessment taxpayers, such as individuals, businesses, and organizations that meet certain income thresholds.
How to fill out AU SA472?
To fill out AU SA472, individuals must gather relevant financial information, complete all sections of the form accurately, and ensure that all required documentation is attached before submitting it.
What is the purpose of AU SA472?
The purpose of AU SA472 is to provide the Australian Taxation Office with a comprehensive overview of an entity's financial situation for accurate assessment of taxes owed.
What information must be reported on AU SA472?
The information that must be reported on AU SA472 includes income details, deductions claimed, any tax credits, and relevant financial records for the assessment year.
Fill out your AU SA472 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

sa472 Form Printable is not the form you're looking for?Search for another form here.
Keywords relevant to australia consent medical information
Related to sa472 consent
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.