Get the free Laboratory Chain of Custody Form Page of
Show details
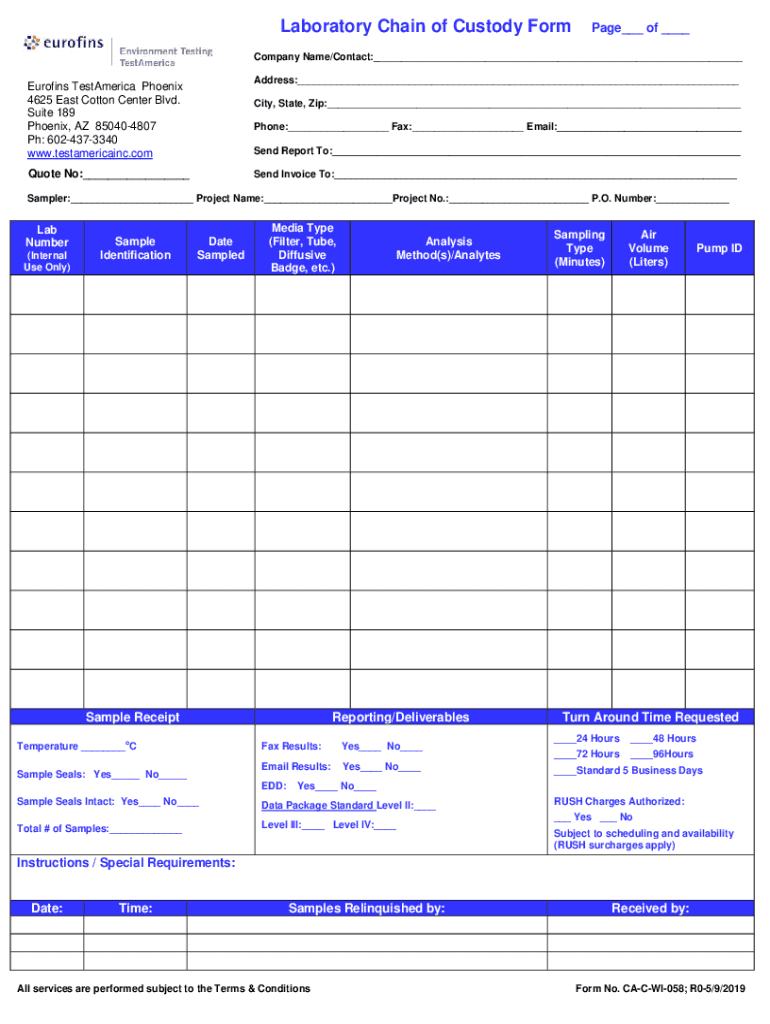
Laboratory Chain of Custody Form Page___ of ___Company Name/Contact:___ Address:___Euro fins Test America Phoenix 4625 East Cotton Center Blvd. Suite 189 Phoenix, AZ 850404807 pH: 6024373340 www.testamericainc.comCity,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign laboratory chain of custody

Edit your laboratory chain of custody form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your laboratory chain of custody form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing laboratory chain of custody online
Follow the guidelines below to take advantage of the professional PDF editor:
1
Log in to account. Click on Start Free Trial and register a profile if you don't have one yet.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit laboratory chain of custody. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out laboratory chain of custody

How to fill out laboratory chain of custody
01
Obtain the chain of custody form from the laboratory.
02
Fill in the requested information accurately, including details such as date, time, location, specimen type, and patient information.
03
Document any handling or transportation details as required, such as sealing the specimen and maintaining the proper temperature.
04
Sign and date the form to certify the accuracy of the information provided.
05
Ensure that the chain of custody form accompanies the specimen throughout the testing process and is securely stored after completion.
Who needs laboratory chain of custody?
01
Individuals or organizations involved in collecting, transporting, testing, or handling specimens for laboratory analysis require a laboratory chain of custody form to maintain a documented record of the specimen's handling and ensure its integrity and legal defensibility.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit laboratory chain of custody from Google Drive?
People who need to keep track of documents and fill out forms quickly can connect PDF Filler to their Google Docs account. This means that they can make, edit, and sign documents right from their Google Drive. Make your laboratory chain of custody into a fillable form that you can manage and sign from any internet-connected device with this add-on.
How do I execute laboratory chain of custody online?
pdfFiller has made it easy to fill out and sign laboratory chain of custody. You can use the solution to change and move PDF content, add fields that can be filled in, and sign the document electronically. Start a free trial of pdfFiller, the best tool for editing and filling in documents.
How can I fill out laboratory chain of custody on an iOS device?
pdfFiller has an iOS app that lets you fill out documents on your phone. A subscription to the service means you can make an account or log in to one you already have. As soon as the registration process is done, upload your laboratory chain of custody. You can now use pdfFiller's more advanced features, like adding fillable fields and eSigning documents, as well as accessing them from any device, no matter where you are in the world.
What is laboratory chain of custody?
Laboratory chain of custody is a documented record that shows the custody, control, transfer, analysis, and disposition of samples in a laboratory.
Who is required to file laboratory chain of custody?
The individuals or organizations responsible for collecting, handling, and analyzing samples are required to file laboratory chain of custody.
How to fill out laboratory chain of custody?
Laboratory chain of custody should be filled out by documenting each step in the sample handling process, including sample collection, transfer, analysis, and disposal.
What is the purpose of laboratory chain of custody?
The purpose of laboratory chain of custody is to ensure the integrity and reliability of sample analysis results by documenting every step in the sample handling process.
What information must be reported on laboratory chain of custody?
Information such as sample collection date and time, collector's name, sample ID, analysis requested, and chain of custody transfer details must be reported on laboratory chain of custody.
Fill out your laboratory chain of custody online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Laboratory Chain Of Custody is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















