Get the free CLINICAL STUDY DOCUMENT APPROVAL FORM Study Name ...
Show details
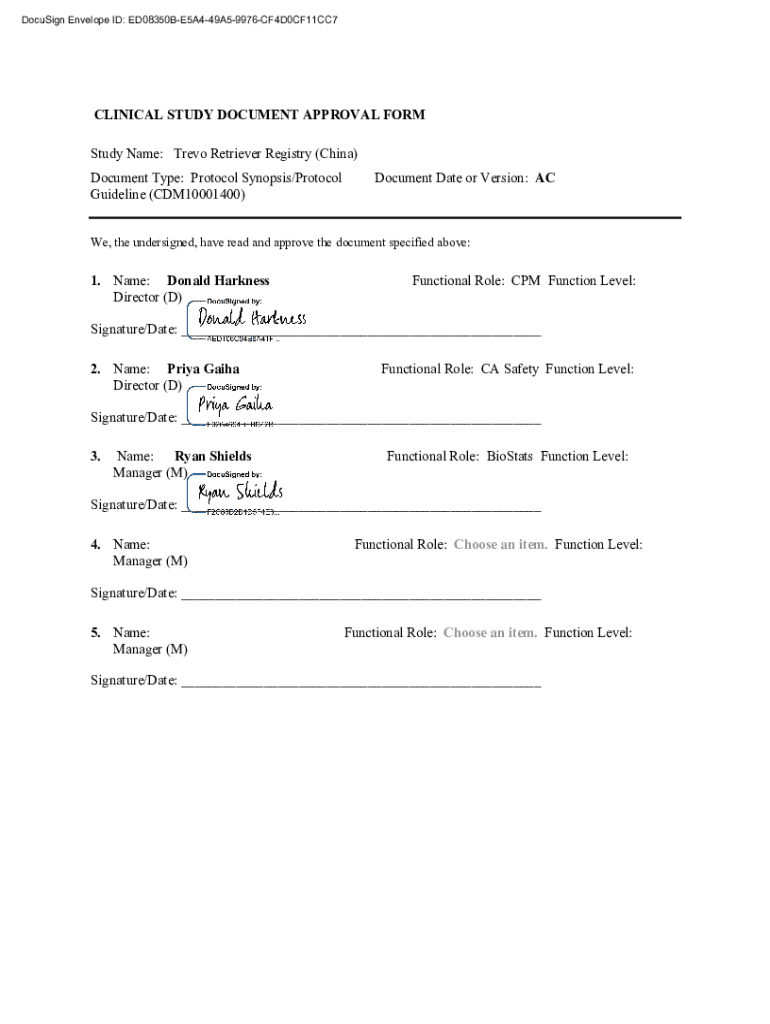
DocuSign Envelope ID: ED08350BE5A449A59976CF4D0CF11CC7CLINICAL STUDY DOCUMENT APPROVAL FORM Study Name: Trevor Retriever Registry (China) Document Type: Protocol Synopsis/Protocol Guideline (CDM10001400)Document
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical study document approval

Edit your clinical study document approval form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical study document approval form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical study document approval online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit clinical study document approval. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical study document approval

How to fill out clinical study document approval
01
Review the specific requirements for the clinical study document approval process.
02
Gather all necessary information and data required for the approval.
03
Fill out all sections of the document accurately and completely.
04
Include any relevant supporting documents or evidence.
05
Double-check the document for errors or missing information before submission.
06
Submit the completed document to the appropriate regulatory authority for review and approval.
Who needs clinical study document approval?
01
Researchers conducting clinical studies
02
Medical professionals overseeing clinical trials
03
Institutional review boards (IRBs) responsible for approving research studies
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my clinical study document approval in Gmail?
In your inbox, you may use pdfFiller's add-on for Gmail to generate, modify, fill out, and eSign your clinical study document approval and any other papers you receive, all without leaving the program. Install pdfFiller for Gmail from the Google Workspace Marketplace by visiting this link. Take away the need for time-consuming procedures and handle your papers and eSignatures with ease.
Can I create an electronic signature for the clinical study document approval in Chrome?
You can. With pdfFiller, you get a strong e-signature solution built right into your Chrome browser. Using our addon, you may produce a legally enforceable eSignature by typing, sketching, or photographing it. Choose your preferred method and eSign in minutes.
How do I complete clinical study document approval on an Android device?
On an Android device, use the pdfFiller mobile app to finish your clinical study document approval. The program allows you to execute all necessary document management operations, such as adding, editing, and removing text, signing, annotating, and more. You only need a smartphone and an internet connection.
What is clinical study document approval?
Clinical study document approval is the process by which regulatory authorities review and approve documents related to a clinical study before it can commence.
Who is required to file clinical study document approval?
The sponsor or principal investigator of the clinical study is required to file for clinical study document approval.
How to fill out clinical study document approval?
Clinical study document approval can be filled out by providing all necessary information and documentation required by the regulatory authorities.
What is the purpose of clinical study document approval?
The purpose of clinical study document approval is to ensure that the study is conducted ethically, safely, and in compliance with regulatory requirements.
What information must be reported on clinical study document approval?
Clinical study document approval typically requires information on study protocol, informed consent forms, investigator's brochure, and other relevant study documents.
Fill out your clinical study document approval online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Study Document Approval is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















