Get the free SERIOUS ADVERSE EVENT FORM
Show details
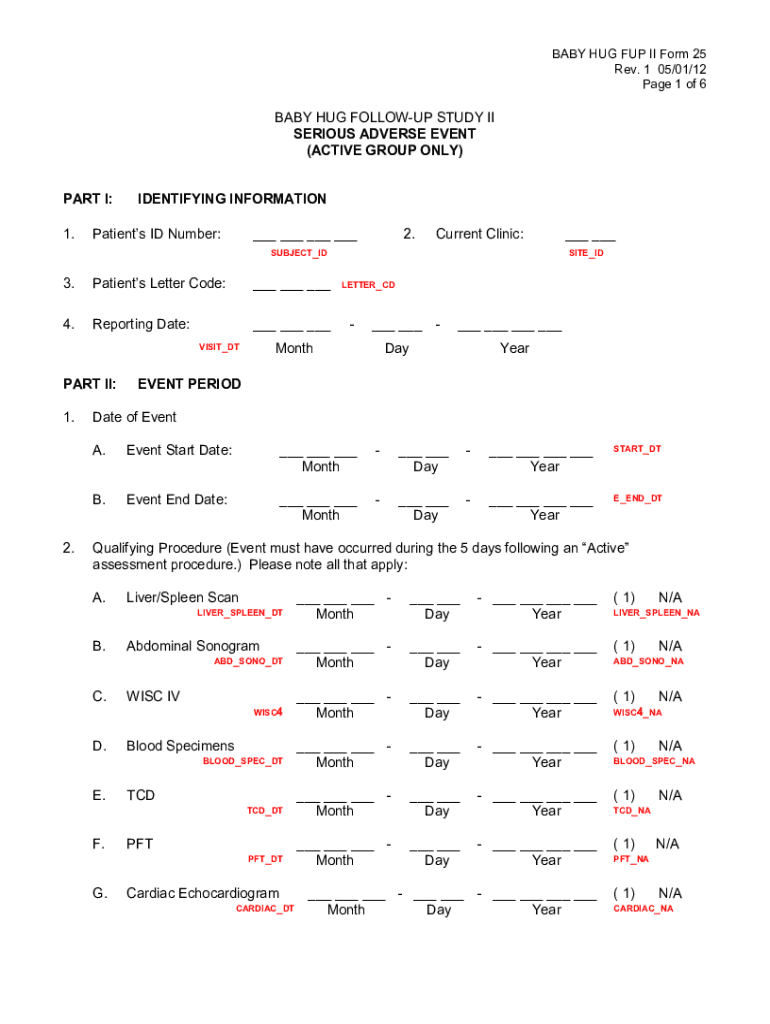
BABY HUG FUP II Form 25
Rev. 1 05/01/12-Page 1 of 6BABY HUG FOLLOWUP STUDY II
SERIOUS ADVERSE EVENT
(ACTIVE GROUP ONLY)
PART I:
1.IDENTIFYING INFORMATIONPatients ID Number:___ ___ ___ ___2. Current
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign serious adverse event form

Edit your serious adverse event form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your serious adverse event form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit serious adverse event form online
To use the services of a skilled PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit serious adverse event form. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
It's easier to work with documents with pdfFiller than you could have ever thought. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out serious adverse event form

How to fill out serious adverse event form
01
Obtain the serious adverse event form from the appropriate medical or regulatory authorities.
02
Fill out the patient's demographics, including name, age, sex, and medical history.
03
Describe the adverse event in detail, including when it occurred, its severity, and any related symptoms.
04
Indicate any medications or treatments the patient was receiving at the time of the event.
05
Include any relevant lab results or diagnostic tests related to the event.
06
Provide contact information for the reporting healthcare provider.
07
Submit the completed form to the required authorities within the specified timeframe.
Who needs serious adverse event form?
01
Healthcare professionals
02
Clinical researchers
03
Medical institutions
04
Pharmaceutical companies
05
Regulatory authorities
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get serious adverse event form?
It's simple using pdfFiller, an online document management tool. Use our huge online form collection (over 25M fillable forms) to quickly discover the serious adverse event form. Open it immediately and start altering it with sophisticated capabilities.
Can I create an eSignature for the serious adverse event form in Gmail?
Use pdfFiller's Gmail add-on to upload, type, or draw a signature. Your serious adverse event form and other papers may be signed using pdfFiller. Register for a free account to preserve signed papers and signatures.
How do I edit serious adverse event form on an Android device?
You can make any changes to PDF files, like serious adverse event form, with the help of the pdfFiller Android app. Edit, sign, and send documents right from your phone or tablet. You can use the app to make document management easier wherever you are.
What is serious adverse event form?
A serious adverse event form is a document used to report any significant negative health event that occurs during a clinical trial or after the administration of a medical product, indicating a potential risk to patients.
Who is required to file serious adverse event form?
Researchers, sponsors of clinical trials, and healthcare providers are typically required to report serious adverse events, particularly when they relate to investigational drugs or devices.
How to fill out serious adverse event form?
To fill out a serious adverse event form, provide detailed information about the patient, event description, severity, outcome, and any causative relationship to the investigational product, along with the date of the event.
What is the purpose of serious adverse event form?
The purpose of the serious adverse event form is to ensure proper documentation, risk assessment, and regulatory compliance related to potential safety issues with medical products.
What information must be reported on serious adverse event form?
The information that must be reported includes patient identification, description of the event, dates of occurrence, relationship to the drug or device, and any actions taken.
Fill out your serious adverse event form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Serious Adverse Event Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















