Get the free FDA and EU GMP Annex 1 Differences in Cleanroom ... - RAPS
Show details
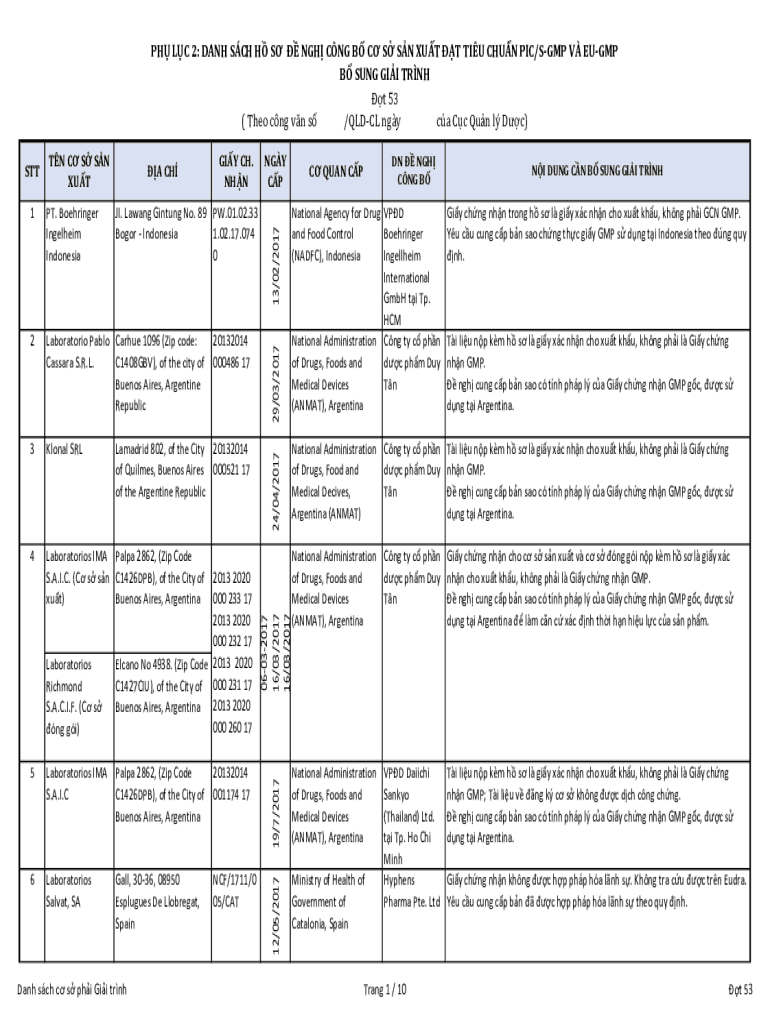
PH LC 2: DAN SCH H S NGC CNG B C S SN BUT T TIE CHEN PIC/SGMP V EU GMP B SUNG GIG TR NH t 53 (Theo CNG VN s /DCL NGC ca Cc Run l Dc)I. Law ang Hinting No. 89 PW.01.02.33 Boor Indonesia 1.02.17.074
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign fda and eu gmp

Edit your fda and eu gmp form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda and eu gmp form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing fda and eu gmp online
To use the services of a skilled PDF editor, follow these steps:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit fda and eu gmp. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, dealing with documents is always straightforward. Try it right now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out fda and eu gmp

How to fill out fda and eu gmp
01
For filling out FDA GMP:
02
Understand the FDA regulations and guidelines for GMP.
03
Gather all necessary information and documentation for compliance.
04
Complete the required forms accurately and thoroughly.
05
Submit the forms to the FDA for review and approval.
06
For filling out EU GMP:
07
Familiarize yourself with the EU GMP regulations and requirements.
08
Ensure that your facility and processes adhere to EU GMP standards.
09
Fill out the necessary documentation and forms according to EU GMP guidelines.
10
Submit the completed forms to the appropriate EU regulatory authority for approval.
Who needs fda and eu gmp?
01
Companies involved in the manufacturing, processing, packaging, or distribution of pharmaceuticals, medical devices, cosmetics, or food products need FDA and EU GMP certification to ensure product quality and safety compliance with regulatory standards.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I sign the fda and eu gmp electronically in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your fda and eu gmp.
How do I edit fda and eu gmp on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share fda and eu gmp from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
How can I fill out fda and eu gmp on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your fda and eu gmp from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is fda and eu gmp?
FDA stands for Food and Drug Administration and EU GMP stands for European Union Good Manufacturing Practice. FDA and EU GMP are regulations that govern the manufacturing and quality control of pharmaceuticals and medical devices.
Who is required to file fda and eu gmp?
Companies involved in manufacturing, importing, distributing, or selling pharmaceuticals or medical devices are required to file FDA and EU GMP.
How to fill out fda and eu gmp?
To fill out FDA and EU GMP, companies must provide detailed information about their manufacturing process, quality control measures, and compliance with regulations.
What is the purpose of fda and eu gmp?
The purpose of FDA and EU GMP is to ensure that pharmaceuticals and medical devices are manufactured in a safe and consistent manner, meeting quality standards and regulations.
What information must be reported on fda and eu gmp?
Information such as manufacturing process, quality control measures, list of ingredients, compliance records, and any adverse events must be reported on FDA and EU GMP.
Fill out your fda and eu gmp online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Fda And Eu Gmp is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















