Get the free Study of Pembrolizumab (MK-3475) vs Standard Therapy in ...
Show details
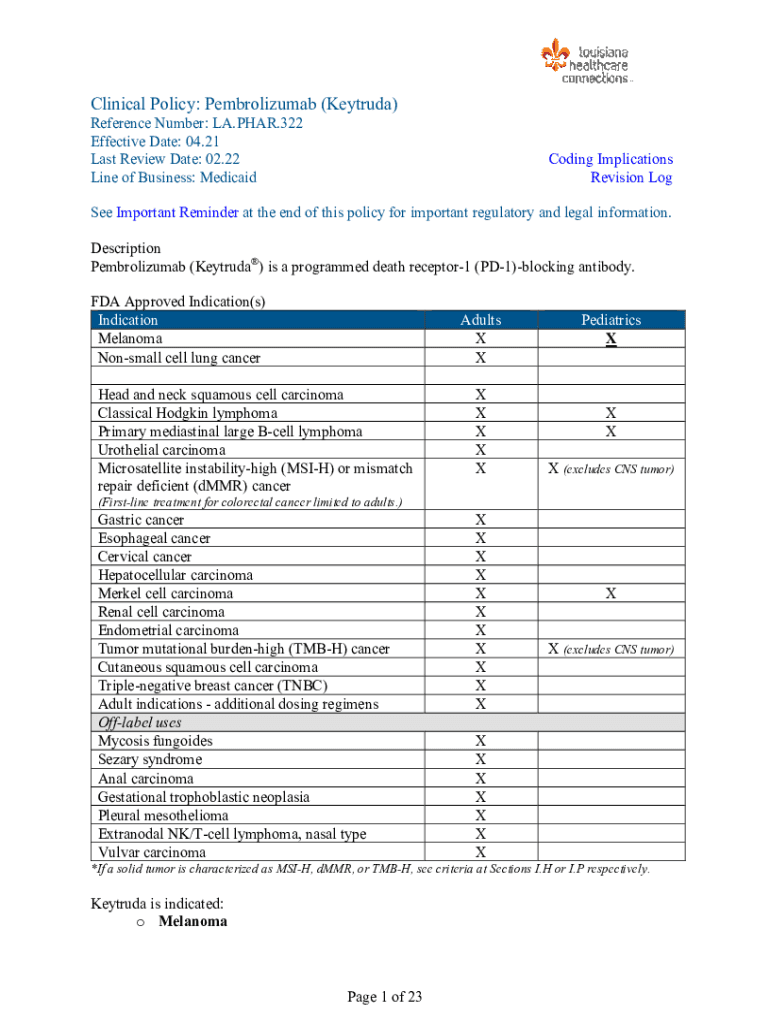
Clinical Policy: Pembrolizumab (Katrina)Reference Number: LA.PAR.322 Effective Date: 04.21 Last Review Date: 02.22 Line of Business: MedicaidCoding Implications Revision Losses Important Reminder
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign study of pembrolizumab mk-3475

Edit your study of pembrolizumab mk-3475 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your study of pembrolizumab mk-3475 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing study of pembrolizumab mk-3475 online
To use the services of a skilled PDF editor, follow these steps below:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit study of pembrolizumab mk-3475. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out study of pembrolizumab mk-3475

How to fill out study of pembrolizumab mk-3475
01
Read the study protocol carefully to understand the requirements and procedures for filling out the study of pembrolizumab mk-3475.
02
Collect all necessary patient information such as medical history, current medications, and previous treatments.
03
Ensure proper documentation of patient consent for participation in the study.
04
Follow the specified instructions for administering pembrolizumab mk-3475 to the patients.
05
Record and report any adverse events or reactions experienced by the patients during the study.
06
Submit all required forms and reports according to the study timeline and guidelines.
Who needs study of pembrolizumab mk-3475?
01
Patients with specific types of cancer such as melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, and Hodgkin lymphoma may benefit from the study of pembrolizumab mk-3475.
02
Researchers and healthcare professionals conducting clinical trials on pembrolizumab mk-3475 may also need to fill out the study to collect data and monitor the treatment's efficacy and safety.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in study of pembrolizumab mk-3475?
With pdfFiller, the editing process is straightforward. Open your study of pembrolizumab mk-3475 in the editor, which is highly intuitive and easy to use. There, you’ll be able to blackout, redact, type, and erase text, add images, draw arrows and lines, place sticky notes and text boxes, and much more.
Can I edit study of pembrolizumab mk-3475 on an iOS device?
Use the pdfFiller mobile app to create, edit, and share study of pembrolizumab mk-3475 from your iOS device. Install it from the Apple Store in seconds. You can benefit from a free trial and choose a subscription that suits your needs.
How do I complete study of pembrolizumab mk-3475 on an iOS device?
Get and install the pdfFiller application for iOS. Next, open the app and log in or create an account to get access to all of the solution’s editing features. To open your study of pembrolizumab mk-3475, upload it from your device or cloud storage, or enter the document URL. After you complete all of the required fields within the document and eSign it (if that is needed), you can save it or share it with others.
What is study of pembrolizumab mk-3475?
The study of pembrolizumab mk-3475 involves researching the effectiveness and safety of the drug in treating various types of cancer.
Who is required to file study of pembrolizumab mk-3475?
Researchers, pharmaceutical companies, or clinical trial sponsors are typically required to file study results of pembrolizumab mk-3475 with regulatory authorities.
How to fill out study of pembrolizumab mk-3475?
The study of pembrolizumab mk-3475 should be filled out according to the guidelines provided by the regulatory authorities, including details on study design, results, and any adverse events.
What is the purpose of study of pembrolizumab mk-3475?
The purpose of the study of pembrolizumab mk-3475 is to gather data on the drug's efficacy, safety, and potential side effects in order to determine its overall benefit-risk profile.
What information must be reported on study of pembrolizumab mk-3475?
Information that must be reported on the study of pembrolizumab mk-3475 includes study protocol, patient demographics, treatment outcomes, adverse events, and statistical analysis.
Fill out your study of pembrolizumab mk-3475 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Study Of Pembrolizumab Mk-3475 is not the form you're looking for?Search for another form here.
Relevant keywords
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















