Get the free Class 2 Device Recall da Vinci Xi EndoWrist Suction Irrigator
Show details
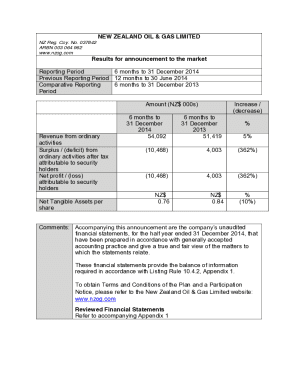
Month Day, Airfield Safety Notice
Urgent Medical Device Correction ISIFA201809C
Intracranial Stops of the da Vinci Xi Endowment Suction IrrigatorDear da Vinci Customer,1 Introduction
and Reason for
Field
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign class 2 device recall

Edit your class 2 device recall form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your class 2 device recall form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit class 2 device recall online
Use the instructions below to start using our professional PDF editor:
1
Log into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit class 2 device recall. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
It's easier to work with documents with pdfFiller than you could have ever thought. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out class 2 device recall

How to fill out class 2 device recall
01
Identify the reason for the recall and gather all necessary information about the device.
02
Notify the appropriate regulatory authorities about the recall and obtain necessary approvals.
03
Inform all affected customers and provide instructions on how to return the devices.
04
Keep detailed records of the recall process, including the number of devices returned and any replacements provided.
05
Monitor and assess the effectiveness of the recall to ensure all affected devices are accounted for.
Who needs class 2 device recall?
01
Manufacturers of class 2 medical devices who have identified a safety issue with their product that could pose a risk to patients or users.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my class 2 device recall in Gmail?
Using pdfFiller's Gmail add-on, you can edit, fill out, and sign your class 2 device recall and other papers directly in your email. You may get it through Google Workspace Marketplace. Make better use of your time by handling your papers and eSignatures.
Can I create an eSignature for the class 2 device recall in Gmail?
Use pdfFiller's Gmail add-on to upload, type, or draw a signature. Your class 2 device recall and other papers may be signed using pdfFiller. Register for a free account to preserve signed papers and signatures.
How do I edit class 2 device recall straight from my smartphone?
The easiest way to edit documents on a mobile device is using pdfFiller’s mobile-native apps for iOS and Android. You can download those from the Apple Store and Google Play, respectively. You can learn more about the apps here. Install and log in to the application to start editing class 2 device recall.
What is class 2 device recall?
A Class 2 device recall is a notification issued by the FDA regarding a medical device that may cause temporary or medically reversible adverse health consequences, or where the probability of serious adverse health consequences is remote.
Who is required to file class 2 device recall?
Manufacturers, importers, and distributors of medical devices are required to file a Class 2 device recall with the FDA.
How to fill out class 2 device recall?
To fill out a Class 2 device recall, a firm must provide a written report to the FDA which includes details such as the device identification, reason for recall, and a strategy for notifying affected parties.
What is the purpose of class 2 device recall?
The purpose of a Class 2 device recall is to protect public health by addressing devices that could cause harm or affect the safety and efficacy of the product.
What information must be reported on class 2 device recall?
Information that must be reported includes the device's name, model number, reason for recall, number of devices affected, and a description of the recall plan.
Fill out your class 2 device recall online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Class 2 Device Recall is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.