Get the free Product Quality Microbiology Information in the
Show details
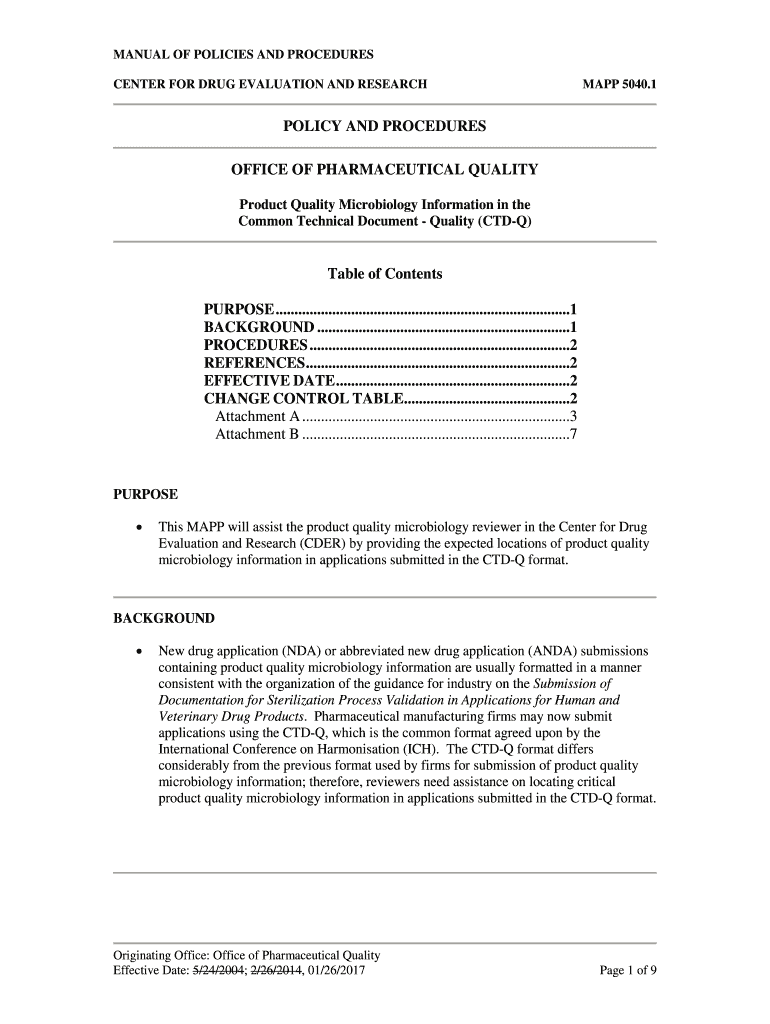
MANUAL OF POLICIES AND PROCEDURES CENTER FOR DRUG EVALUATION AND ResearchGate 5040.1POLICY AND PROCEDURES OFFICE OF PHARMACEUTICAL QUALITY Product Quality Microbiology Information in the Common Technical
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign product quality microbiology information

Edit your product quality microbiology information form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your product quality microbiology information form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing product quality microbiology information online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit product quality microbiology information. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out product quality microbiology information

How to fill out product quality microbiology information?
01
Start by identifying the specific product for which you are collecting microbiology information. This can include food products, pharmaceuticals, cosmetics, or any other items that may have microbiological concerns.
02
Gather all necessary information about the product, such as its ingredients, manufacturing process, and intended use. This will help in determining the potential sources of microbiological contamination and the specific tests that need to be conducted.
03
Determine the applicable regulatory requirements for microbiological testing. Different industries and regions may have specific guidelines and limits for microbial counts or the presence of certain pathogens. Ensure that you are familiar with these requirements to ensure compliance.
04
Select the appropriate microbiological testing methods based on the specific product and regulatory guidelines. This may involve culturing samples on different culture media, conducting PCR testing, or using rapid methods for detection.
05
Follow standard operating procedures (SOPs) for sample collection, preparation, and testing. The SOPs should include detailed instructions on sample size, preservation, transportation, and other factors that may impact microbiological results. Adhering to these procedures will help ensure accurate and reliable data.
06
Maintain detailed records of the microbiological testing process. This includes documenting the sample collection date, testing date, testing method, and any observations or deviations from the standard procedures. These records will be valuable for future reference and may be required for audits or regulatory purposes.
Who needs product quality microbiology information?
01
Manufacturers: Product quality microbiology information is essential for manufacturers to ensure that their products meet the necessary safety and quality standards. This information helps in identifying potential sources of contamination, implementing corrective actions, and preventing product recalls.
02
Regulatory authorities: Regulatory agencies rely on product quality microbiology information to assess the safety and compliance of products in the market. This information helps in establishing guidelines, setting limits for microbial counts, and monitoring product quality and safety.
03
Consumers: Ultimately, consumers are the end-users of the products and need to have confidence in their safety. Knowing that product quality microbiology information has been collected and analyzed ensures that consumers can make informed choices and trust the products they are using.
In conclusion, filling out product quality microbiology information requires a systematic approach that involves gathering relevant information, selecting appropriate testing methods, following standard procedures, and maintaining accurate records. This information is crucial for manufacturers, regulatory authorities, and consumers alike to ensure product safety and quality.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is product quality microbiology information?
Product quality microbiology information refers to the data and analysis regarding the microbial content and quality of a product.
Who is required to file product quality microbiology information?
Manufacturers and producers of products are required to file product quality microbiology information.
How to fill out product quality microbiology information?
Product quality microbiology information can be filled out by collecting relevant data and submitting it through the designated channels provided by the regulatory authorities.
What is the purpose of product quality microbiology information?
The purpose of product quality microbiology information is to ensure the safety and quality of products by monitoring and analyzing their microbial content.
What information must be reported on product quality microbiology information?
Information such as microbial tests results, contamination levels, and quality control measures must be reported on product quality microbiology information.
Where do I find product quality microbiology information?
The premium pdfFiller subscription gives you access to over 25M fillable templates that you can download, fill out, print, and sign. The library has state-specific product quality microbiology information and other forms. Find the template you need and change it using powerful tools.
How do I edit product quality microbiology information on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share product quality microbiology information from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
Can I edit product quality microbiology information on an Android device?
Yes, you can. With the pdfFiller mobile app for Android, you can edit, sign, and share product quality microbiology information on your mobile device from any location; only an internet connection is needed. Get the app and start to streamline your document workflow from anywhere.
Fill out your product quality microbiology information online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Product Quality Microbiology Information is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















