Get the free Investigator Sponsored Research - 3M Healthcare
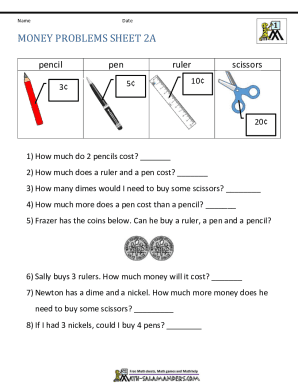
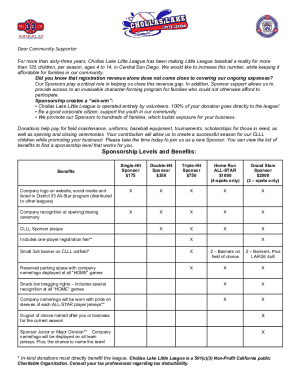
Show details
HEALTHCARE sELfsTudy sERiEs EWs PuRCHAsing nOctober 2018The selfstudy lesson on this central service topic was developed by 3M Health Care. The lessons are administered by Endeavor Healthcare MediaEarn
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign investigator sponsored research

Edit your investigator sponsored research form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your investigator sponsored research form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing investigator sponsored research online
Follow the steps down below to benefit from the PDF editor's expertise:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit investigator sponsored research. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out investigator sponsored research

How to fill out investigator sponsored research
01
Obtain the necessary application forms and guidelines for investigator sponsored research from the relevant institution or funding agency.
02
Review the application forms and guidelines thoroughly to understand the requirements, eligibility criteria, and submission deadlines.
03
Gather all the necessary information and documentation required for the research project, such as project proposal, budget details, timeline, and ethical considerations.
04
Fill out the application form accurately and completely, ensuring all the requested information is provided, and any additional materials are attached as required.
05
Double-check the completed application form for any errors or missing information before submitting.
06
Prepare all supporting documents and ensure they are organized and properly labeled.
07
Submit the completed application and supporting documents as per the submission guidelines and deadlines specified.
08
Await the decision from the institution or funding agency regarding the approval of the investigator sponsored research project.
09
If approved, comply with any additional requirements or conditions specified by the institution or funding agency.
10
Begin the research project and ensure regular progress updates and reporting as required.
11
Adhere to all ethical guidelines and regulations throughout the research process.
12
Upon completion of the research project, provide the institution or funding agency with any final reports or findings as requested.
Who needs investigator sponsored research?
01
Investigator sponsored research is needed by individuals or organizations conducting independent research studies.
02
Academic researchers and scientists often require investigator sponsored research to gather data, conduct experiments, or test hypotheses.
03
Pharmaceutical companies and medical device manufacturers may engage in investigator sponsored research to evaluate the safety and efficacy of their products.
04
Government agencies and non-profit organizations may rely on investigator sponsored research to gather evidence and support policy-making decisions.
05
Healthcare institutions and hospitals may participate in investigator sponsored research to contribute to medical advancements and improve patient care.
06
Biotechnology and biomedical companies may utilize investigator sponsored research to explore new treatments or technologies.
07
Investor groups and venture capitalists may be interested in investigator sponsored research to evaluate potential investment opportunities.
08
Overall, anyone who seeks to advance scientific knowledge, improve healthcare practices, or explore new innovations may benefit from investigator sponsored research.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit investigator sponsored research online?
pdfFiller not only lets you change the content of your files, but you can also change the number and order of pages. Upload your investigator sponsored research to the editor and make any changes in a few clicks. The editor lets you black out, type, and erase text in PDFs. You can also add images, sticky notes, and text boxes, as well as many other things.
How can I edit investigator sponsored research on a smartphone?
Using pdfFiller's mobile-native applications for iOS and Android is the simplest method to edit documents on a mobile device. You may get them from the Apple App Store and Google Play, respectively. More information on the apps may be found here. Install the program and log in to begin editing investigator sponsored research.
Can I edit investigator sponsored research on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share investigator sponsored research from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
What is investigator sponsored research?
Investigator sponsored research refers to studies that are initiated and conducted by an individual researcher rather than a pharmaceutical company or sponsor. These studies often involve new drugs or interventions and are typically aimed at gathering data on safety, efficacy, or outcomes.
Who is required to file investigator sponsored research?
Researchers or investigators who initiate and conduct their own studies, particularly those that involve human subjects or new treatments, are required to file investigator sponsored research.
How to fill out investigator sponsored research?
Filling out investigator sponsored research typically involves submitting a detailed proposal that includes the study design, methodology, research objectives, informed consent procedures, and any external funding sources. It's important to follow institutional guidelines and regulatory requirements.
What is the purpose of investigator sponsored research?
The purpose of investigator sponsored research is to generate new knowledge, evaluate new interventions, or improve existing treatments. This type of research often addresses questions that are not being explored by commercial sponsors.
What information must be reported on investigator sponsored research?
Information that must be reported includes study design, objectives, methodology, data collection processes, ethical considerations, conflict of interest disclosures, and results of the research.
Fill out your investigator sponsored research online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Investigator Sponsored Research is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.