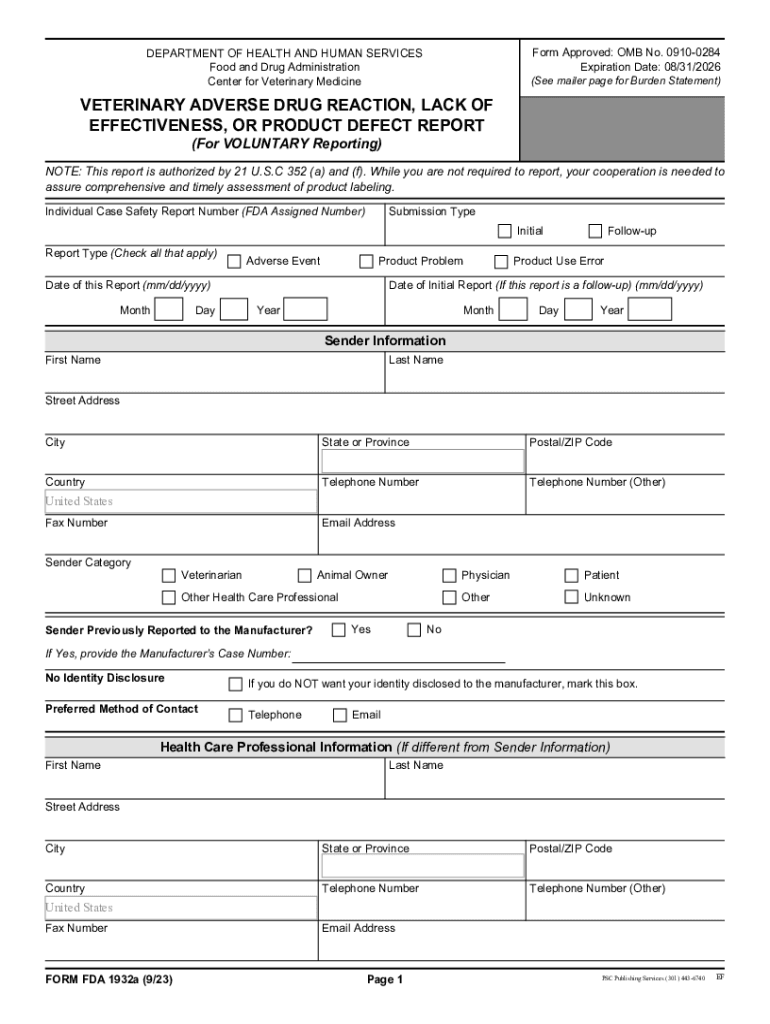
HHS Form FDA 1932a 2023-2025 free printable template
Show details
DESCRIBE THE REACTION ADD DETAILS ABOUT CASE HISTORY AND OUTCOME Include numbers if group of animals involved GIVE COMMENT ON POSSIBLE CONTRIBUTING FACTORS. DESCRIBE LACK OF EFFECTIVENESS OR PRODUCT DEFECT Include Expiration Date and Lot No. NOTE Triple fold as marked seal with tape no postage required additional space on back if needed. FORM FDA 1932a 3/07 PSC Graphics 301 443-1090 EF An agency may not conduct or sponsor and a person is not requ...
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign HHS Form FDA 1932a

Edit your HHS Form FDA 1932a form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your HHS Form FDA 1932a form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit HHS Form FDA 1932a online
To use our professional PDF editor, follow these steps:
1
Log into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit HHS Form FDA 1932a. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
It's easier to work with documents with pdfFiller than you could have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
HHS Form FDA 1932a Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out HHS Form FDA 1932a

How to fill out HHS Form FDA 1932a
01
Obtain the HHS Form FDA 1932a from the official FDA website.
02
Fill out the appellant's name and contact information at the top of the form.
03
Provide relevant details about the product involved, including its name and description.
04
Specify the type of application or submission being contested.
05
Clearly state the basis for the appeal, including any facts and legal arguments.
06
Include any supportive documentation or evidence related to the appeal.
07
Sign and date the form at the required section before submission.
08
Submit the completed form to the appropriate FDA office as indicated in the instructions.
Who needs HHS Form FDA 1932a?
01
Individuals or organizations that wish to appeal a decision made by the FDA regarding a product or application need HHS Form FDA 1932a.
Fill
form
: Try Risk Free






People Also Ask about
What is the FDA adverse event reporting form?
Form FDA 3500 may be used by health professionals or consumers for VOLUNTARY reporting of adverse events, product use/medication errors, product quality problems, and therapeutic failures for: Prescription and over-the-counter medicines including those administered in a hospital or outpatient infusion centers.
What are the FDA requirements for reporting adverse events?
The event is serious and should be reported to FDA when the patient outcome is: Death. Life-threatening. Hospitalization (initial or prolonged) Disability or Permanent Damage. Congenital Anomaly/Birth Defect. Required Intervention to Prevent Permanent Impairment or Damage (Devices) Other Serious (Important Medical Events)
Do veterinary drugs require FDA approval?
Animal Drugs– The Federal Food, Drug, and Cosmetic Act gives FDA the legal authority to approve and regulate drugs for animals. Before a drug company can market an animal drug, the company must get the drug approved by FDA.
How do I report a medication adverse event?
Call FDA at 1-800-FDA-1088 to report by telephone. Reporting Form FDA 3500 commonly used by health professionals.
How do I report a drug problem to the FDA?
To report an emergency involving food, drugs, medical devices, dietary supplements, or cosmetics, call 1-866-300-4374 or 1-301-796-8240.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an eSignature for the HHS Form FDA 1932a in Gmail?
You may quickly make your eSignature using pdfFiller and then eSign your HHS Form FDA 1932a right from your mailbox using pdfFiller's Gmail add-on. Please keep in mind that in order to preserve your signatures and signed papers, you must first create an account.
How can I edit HHS Form FDA 1932a on a smartphone?
You can easily do so with pdfFiller's apps for iOS and Android devices, which can be found at the Apple Store and the Google Play Store, respectively. You can use them to fill out PDFs. We have a website where you can get the app, but you can also get it there. When you install the app, log in, and start editing HHS Form FDA 1932a, you can start right away.
How do I fill out HHS Form FDA 1932a on an Android device?
Use the pdfFiller Android app to finish your HHS Form FDA 1932a and other documents on your Android phone. The app has all the features you need to manage your documents, like editing content, eSigning, annotating, sharing files, and more. At any time, as long as there is an internet connection.
What is HHS Form FDA 1932a?
HHS Form FDA 1932a is a form used to report adverse events related to medical products regulated by the FDA, specifically for FDA-regulated products such as drugs, biologics and devices.
Who is required to file HHS Form FDA 1932a?
Any healthcare professional, patient, or consumer who experiences an adverse event from an FDA-regulated product, as well as manufacturers and sponsors of these products, are required to file HHS Form FDA 1932a.
How to fill out HHS Form FDA 1932a?
To fill out HHS Form FDA 1932a, you need to provide detailed information about the adverse event, including the product involved, patient information, a description of the event, and any relevant medical history. The form can be completed online or by hand.
What is the purpose of HHS Form FDA 1932a?
The purpose of HHS Form FDA 1932a is to collect information on adverse events in order to monitor the safety of FDA-regulated products and to identify potential safety issues that may require regulatory action.
What information must be reported on HHS Form FDA 1932a?
On HHS Form FDA 1932a, the following information must be reported: details of the adverse event, product information (including name and manufacturer), patient demographics, the reporter's contact information, and any other necessary medical history relevant to the event.
Fill out your HHS Form FDA 1932a online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

HHS Form FDA 1932a is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.























