Get the free Schedule I-II Controlled Substances
Show details
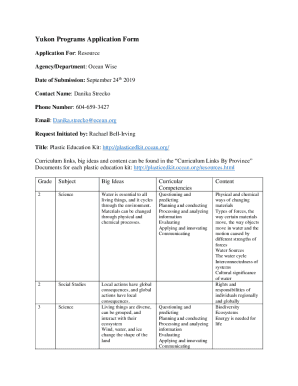
Controlled Substances Disposal Instructions Schedule I-II Controlled Substances To disposal of Schedule I or II drugs, pre-approval is required by the disposal Subcontractor, EXP Pharmaceutical, Inc.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign schedule i-ii controlled substances

Edit your schedule i-ii controlled substances form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your schedule i-ii controlled substances form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit schedule i-ii controlled substances online
Follow the steps down below to benefit from the PDF editor's expertise:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit schedule i-ii controlled substances. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out schedule i-ii controlled substances

How to fill out schedule I-II controlled substances:
01
Obtain the correct form: To fill out schedule I-II controlled substances, you need to obtain and use the specific form provided by the designated authorities. Typically, this form is known as the DEA Form 222 or the Controlled Substance Ordering System (CSOS) electronic order form.
02
Fill in the supplier information: Start by entering the supplier's information accurately on the form. Include the supplier's name, address, and DEA registration number. This information ensures that the controlled substances are obtained from a legitimate source.
03
Provide the recipient information: Fill out the recipient information, which includes the name, address, and DEA registration number of the location or person receiving the controlled substances. Ensure the accuracy of this information to prevent any potential errors or complications.
04
Complete the item section: In this section, list the specific controlled substances being ordered. Include the name, strength, dosage form, and quantity of each controlled substance. Ensure the accuracy of this information and double-check it against the prescribing information to avoid any mistakes.
05
Record the number of containers: Specify the number of containers for each controlled substance being ordered. This helps in accurately tracking and accounting for the quantities of controlled substances involved.
06
Calculate the total quantity: Calculate the total quantity of each controlled substance by multiplying the number of containers by the quantity per container. This ensures that the ordered amount matches the intended amount without any discrepancies.
07
Include the date: Don't forget to include the date of the order on the form, as this information is crucial for record-keeping purposes.
Who needs schedule I-II controlled substances?
01
Medical professionals: Physicians, dentists, veterinarians, and other authorized healthcare practitioners may need schedule I-II controlled substances for specific medical treatments and interventions. They must adhere to strict regulations and prescribing practices to ensure safe and responsible use.
02
Pharmacies and distributors: Licensed pharmacies and distributors require schedule I-II controlled substances to fill prescriptions and supply medications to healthcare professionals. They must comply with legal requirements regarding storage, handling, and distribution of these substances.
03
Research institutions: Universities, research facilities, and scientific organizations may require schedule I-II controlled substances for research and experimentation purposes. Proper protocols and permits should be obtained to ensure compliance with regulations and ethical standards.
In conclusion, filling out schedule I-II controlled substances involves obtaining the correct form, providing supplier and recipient information accurately, completing the item section, recording the number of containers, calculating the total quantity, and including the date. These controlled substances are needed by medical professionals, pharmacies and distributors, and research institutions for various lawful purposes.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is schedule i-ii controlled substances?
Schedule I-II controlled substances are drugs or substances that are regulated by the government due to their high potential for abuse and addiction.
Who is required to file schedule i-ii controlled substances?
Certain healthcare providers, pharmacies, and manufacturers who handle schedule I-II controlled substances are required to file reports with the appropriate regulatory agency.
How to fill out schedule i-ii controlled substances?
To fill out schedule I-II controlled substances, the provider must accurately document the quantity, dosage, and patient information for each controlled substance dispensed or administered.
What is the purpose of schedule i-ii controlled substances?
The purpose of regulating schedule I-II controlled substances is to prevent abuse, diversion, and misuse of highly addictive drugs.
What information must be reported on schedule i-ii controlled substances?
The information required to be reported on schedule I-II controlled substances includes the name of the drug, the quantity dispensed, the date of dispensing, and the patient's information.
Can I create an electronic signature for signing my schedule i-ii controlled substances in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your schedule i-ii controlled substances and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
How do I fill out the schedule i-ii controlled substances form on my smartphone?
The pdfFiller mobile app makes it simple to design and fill out legal paperwork. Complete and sign schedule i-ii controlled substances and other papers using the app. Visit pdfFiller's website to learn more about the PDF editor's features.
How do I edit schedule i-ii controlled substances on an iOS device?
You can. Using the pdfFiller iOS app, you can edit, distribute, and sign schedule i-ii controlled substances. Install it in seconds at the Apple Store. The app is free, but you must register to buy a subscription or start a free trial.
Fill out your schedule i-ii controlled substances online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Schedule I-Ii Controlled Substances is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.