Get the free Critical DatesPharmaceutical Outcomes & Policy
Show details
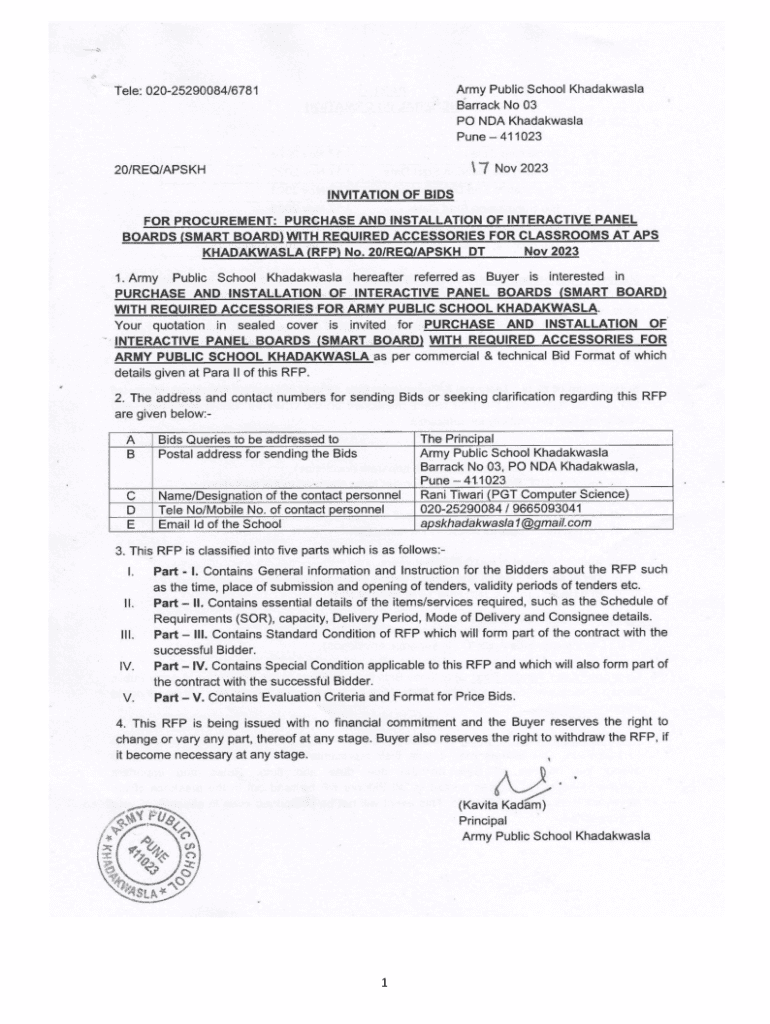
1PART I GENERAL INFORMATION 1. Critical Dates: APublishing Date17 Nov 2023BSeeking Clarification Start Date17 Nov 2023CClarification End Date21 Nov 2023DBid Submission Start Date17 Nov 2023EBid Submission
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign critical datespharmaceutical outcomes amp

Edit your critical datespharmaceutical outcomes amp form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your critical datespharmaceutical outcomes amp form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing critical datespharmaceutical outcomes amp online
To use the professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit critical datespharmaceutical outcomes amp. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out critical datespharmaceutical outcomes amp

How to fill out critical datespharmaceutical outcomes amp
01
To fill out critical dates pharmaceutical outcomes AMP, follow these steps:
02
Start by gathering all the necessary information related to pharmaceutical outcomes AMP, including the critical dates.
03
Identify the specific pharmaceutical outcomes that need to be recorded and tracked using critical dates AMP.
04
Create a detailed record of each critical date, including the date, time, and relevant details or actions associated with it.
05
Update the critical dates AMP regularly to ensure accuracy and timeliness of information.
06
Provide clear and concise documentation for each pharmaceutical outcome recorded in the critical dates AMP.
07
Review and validate the critical dates periodically to ensure consistency and adherence to compliance regulations.
08
Use appropriate software or tools to manage and organize the critical dates pharmaceutical outcomes AMP efficiently.
09
Train the relevant personnel on how to access, update, and interpret the critical dates AMP to facilitate proper decision-making and planning.
10
Maintain a backup or redundant system to prevent data loss or corruption of the critical dates pharmaceutical outcomes AMP.
11
Continuously monitor and evaluate the effectiveness of the critical dates AMP to identify any areas for improvement.
Who needs critical datespharmaceutical outcomes amp?
01
Various stakeholders within the pharmaceutical industry and related fields may require critical dates pharmaceutical outcomes AMP, including:
02
- Pharmaceutical companies, to track and manage critical dates associated with drug development and regulatory compliance.
03
- Research organizations or institutions, to record critical dates related to clinical trials and research studies.
04
- Regulatory agencies, to monitor and enforce compliance with pharmaceutical regulations and deadlines.
05
- Healthcare professionals, to stay updated on critical dates for drug approvals, recalls, or safety notifications.
06
- Legal departments or organizations involved in pharmaceutical litigation or intellectual property rights.
07
- Patients or healthcare consumers who want to track drug expiration dates or medication usage guidelines.
08
- Insurance companies or healthcare providers, to manage prescription drug coverage and reimbursement timelines.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make edits in critical datespharmaceutical outcomes amp without leaving Chrome?
Adding the pdfFiller Google Chrome Extension to your web browser will allow you to start editing critical datespharmaceutical outcomes amp and other documents right away when you search for them on a Google page. People who use Chrome can use the service to make changes to their files while they are on the Chrome browser. pdfFiller lets you make fillable documents and make changes to existing PDFs from any internet-connected device.
How can I edit critical datespharmaceutical outcomes amp on a smartphone?
Using pdfFiller's mobile-native applications for iOS and Android is the simplest method to edit documents on a mobile device. You may get them from the Apple App Store and Google Play, respectively. More information on the apps may be found here. Install the program and log in to begin editing critical datespharmaceutical outcomes amp.
How do I edit critical datespharmaceutical outcomes amp on an Android device?
You can. With the pdfFiller Android app, you can edit, sign, and distribute critical datespharmaceutical outcomes amp from anywhere with an internet connection. Take use of the app's mobile capabilities.
What is critical datespharmaceutical outcomes amp?
Critical dates pharmaceutical outcomes amp refers to the key timelines and results related to the approval and monitoring of pharmaceutical products, impacting their availability and usage in healthcare.
Who is required to file critical datespharmaceutical outcomes amp?
Pharmaceutical companies, manufacturers, and other stakeholders involved in drug development and commercialization are required to file critical dates pharmaceutical outcomes.
How to fill out critical datespharmaceutical outcomes amp?
To fill out critical dates pharmaceutical outcomes, stakeholders should provide specific details regarding timelines, results, and events related to the pharmaceutical product, ensuring accuracy and completeness in the submission.
What is the purpose of critical datespharmaceutical outcomes amp?
The purpose of critical dates pharmaceutical outcomes is to ensure transparency and accountability in drug development, convey important milestones, and facilitate monitoring by regulatory authorities.
What information must be reported on critical datespharmaceutical outcomes amp?
Information such as initiation and completion dates of clinical trials, submission dates for regulatory reviews, and outcomes of those submissions must be reported in critical dates pharmaceutical outcomes.
Fill out your critical datespharmaceutical outcomes amp online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Critical Datespharmaceutical Outcomes Amp is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















