Get the free Clinical Review - Chlormethine Gel (Ledaga)
Show details
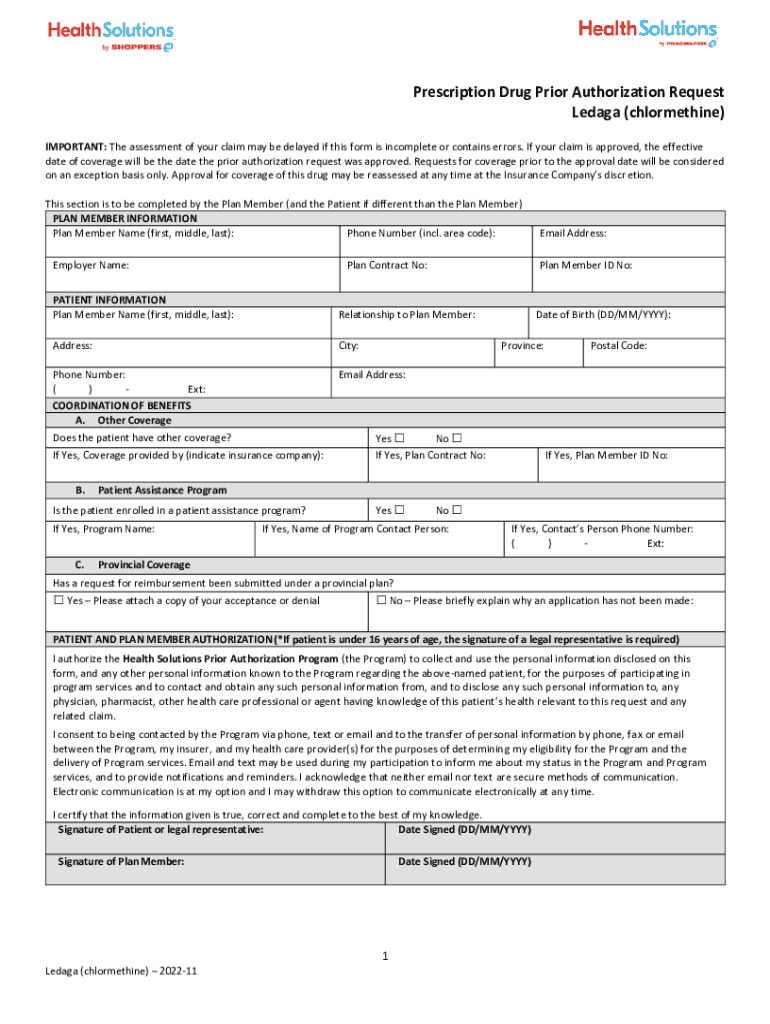
Prescription Drug Prior Authorization Request Ledaga (chlormethine) IMPORTANT: The assessment of your claim may be delayed if this form is incomplete or contains errors. If your claim is approved,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical review - chlormethine

Edit your clinical review - chlormethine form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical review - chlormethine form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical review - chlormethine online
Follow the steps below to benefit from the PDF editor's expertise:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit clinical review - chlormethine. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical review - chlormethine

How to fill out clinical review - chlormethine
01
To fill out a clinical review for chlormethine, follow these steps:
02
Start by gathering all relevant and necessary patient information, including medical history and current condition.
03
Review the prescribed dosage and administration instructions for chlormethine.
04
Evaluate the patient's suitability for chlormethine treatment based on their medical history, current condition, and any contraindications or precautions associated with the medication.
05
Document any previous treatments or medications the patient has tried, their effectiveness, and any adverse reactions.
06
Assess the patient's response to chlormethine if they have started the treatment already.
07
Consider any potential drug interactions with chlormethine and other medications the patient is currently taking.
08
Provide a comprehensive analysis of the patient's overall health condition and the benefits and risks of using chlormethine.
09
Include any additional recommendations or alternative treatment options if applicable.
10
Complete the clinical review by summarizing the findings and providing a clear and concise treatment plan for the patient.
11
Ensure to follow any specific guidelines or requirements set by the healthcare facility or regulatory authorities when filling out the clinical review for chlormethine.
Who needs clinical review - chlormethine?
01
Clinical review for chlormethine is typically required for patients who:
02
- Have been prescribed chlormethine for the treatment of their medical condition
03
- Have a complex medical history or pre-existing conditions that may impact the safety or effectiveness of chlormethine
04
- Require close monitoring or follow-up due to potential risks associated with chlormethine
05
- Need a comprehensive evaluation of their overall health condition and treatment options before starting chlormethine
06
- Have experienced adverse reactions or insufficient response to previous treatments or medications
07
- Are part of a clinical trial or research study involving chlormethine
08
- Are being considered for off-label use of chlormethine
09
- Are suspected to have contraindications or potential drug interactions with chlormethine
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit clinical review - chlormethine from Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your clinical review - chlormethine into a dynamic fillable form that can be managed and signed using any internet-connected device.
How can I send clinical review - chlormethine for eSignature?
Once your clinical review - chlormethine is complete, you can securely share it with recipients and gather eSignatures with pdfFiller in just a few clicks. You may transmit a PDF by email, text message, fax, USPS mail, or online notarization directly from your account. Make an account right now and give it a go.
How do I execute clinical review - chlormethine online?
With pdfFiller, you may easily complete and sign clinical review - chlormethine online. It lets you modify original PDF material, highlight, blackout, erase, and write text anywhere on a page, legally eSign your document, and do a lot more. Create a free account to handle professional papers online.
What is clinical review - chlormethine?
Clinical review - chlormethine refers to the evaluation of clinical data related to chlormethine, a chemotherapy medication, to assess its safety, efficacy, and regulatory compliance.
Who is required to file clinical review - chlormethine?
Manufacturers, sponsors of clinical trials, and healthcare providers involved in the administration or study of chlormethine are typically required to file a clinical review.
How to fill out clinical review - chlormethine?
To fill out the clinical review for chlormethine, gather necessary clinical trial data, complete the prescribed forms with accurate information, and submit them to the relevant regulatory authority.
What is the purpose of clinical review - chlormethine?
The purpose of the clinical review - chlormethine is to ensure that the medication's safety and efficacy are thoroughly evaluated before it can be approved for use.
What information must be reported on clinical review - chlormethine?
The report must include clinical trial data, adverse event reports, dosage information, treatment outcomes, and details regarding patient demographics.
Fill out your clinical review - chlormethine online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Review - Chlormethine is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















