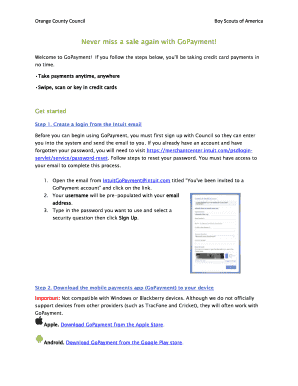
Get the free Clinical Trials Ontario Conference Toronto
Show details
661 University Avenue, Suite 460
MaRS Center, West Tower
Toronto, Ontario
M5G 1M1 Canada
www.ctontario.caDocumented Institutional Ethics Requirements
Cardio Health Clinical Trials
Missions and Values
Provide
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical trials ontario conference

Edit your clinical trials ontario conference form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical trials ontario conference form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical trials ontario conference online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit clinical trials ontario conference. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
It's easier to work with documents with pdfFiller than you can have believed. You can sign up for an account to see for yourself.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical trials ontario conference

How to fill out clinical trials ontario conference
01
Start by downloading the conference registration form from the official Clinical Trials Ontario website.
02
Fill out your personal information, including your name, contact details, and job title.
03
Specify if you are attending as an individual or as part of a group.
04
Provide any dietary restrictions or special accommodations you may require during the conference.
05
Select the type of registration package you wish to purchase, such as full conference access or single day attendance.
06
Indicate if you will be presenting any research or participating in any sessions as a speaker.
07
If applicable, provide the names and affiliations of any co-presenters or co-authors.
08
Read and agree to the terms and conditions of the conference, including cancellation policies and photography consent.
09
Double-check all the information you have filled out for accuracy and completeness.
10
Submit the completed registration form along with any required payment to the designated contact or email address.
11
Await confirmation of your registration from the conference organizers.
12
Make necessary arrangements for travel, accommodation, and other logistics related to attending the Clinical Trials Ontario conference.
Who needs clinical trials ontario conference?
01
Clinical Trials Ontario conference is beneficial for a wide range of individuals and organizations involved in clinical research and trials. This includes:
02
- Researchers and scientists looking to share their findings and learn about the latest developments in the field.
03
- Healthcare professionals interested in expanding their knowledge and skills related to clinical trials.
04
- Pharmaceutical and biotech companies seeking to network with other industry professionals and explore potential collaborations.
05
- Regulatory authorities and policymakers involved in shaping the regulations and policies governing clinical trials.
06
- Patient advocacy groups and patient representatives who play a crucial role in ensuring patient-centered research.
07
- Ethics committee members responsible for reviewing and approving clinical trial protocols.
08
- Research coordinators and clinical trial investigators involved in the management and execution of trials.
09
- Data managers and statisticians responsible for analyzing and interpreting clinical trial data.
10
- Students and trainees interested in pursuing a career in clinical research and trials.
11
- Anyone with a general interest in the field of clinical trials and a desire to stay informed about the latest advancements and best practices.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete clinical trials ontario conference online?
pdfFiller has made filling out and eSigning clinical trials ontario conference easy. The solution is equipped with a set of features that enable you to edit and rearrange PDF content, add fillable fields, and eSign the document. Start a free trial to explore all the capabilities of pdfFiller, the ultimate document editing solution.
Can I create an eSignature for the clinical trials ontario conference in Gmail?
You can easily create your eSignature with pdfFiller and then eSign your clinical trials ontario conference directly from your inbox with the help of pdfFiller’s add-on for Gmail. Please note that you must register for an account in order to save your signatures and signed documents.
How do I fill out clinical trials ontario conference using my mobile device?
Use the pdfFiller mobile app to fill out and sign clinical trials ontario conference. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, their features, and how to get started.
What is clinical trials ontario conference?
The Clinical Trials Ontario Conference is an event focused on bringing together stakeholders involved in clinical trials to discuss advancements, share knowledge, and enhance collaboration in the clinical research landscape in Ontario.
Who is required to file clinical trials ontario conference?
All sponsors and investigators conducting clinical trials in Ontario are typically required to file the necessary documentation associated with the Clinical Trials Ontario Conference.
How to fill out clinical trials ontario conference?
To fill out the Clinical Trials Ontario Conference forms, attendees should gather the necessary information about their clinical trials, ensure all required sections are completed accurately, and submit the forms through the designated online portal or submission method outlined by the conference organizers.
What is the purpose of clinical trials ontario conference?
The purpose of the Clinical Trials Ontario Conference is to provide a platform for discussion and knowledge exchange, promote best practices in clinical trials, and facilitate networking among researchers, sponsors, regulatory bodies, and other stakeholders.
What information must be reported on clinical trials ontario conference?
Participants must report information including trial design, sponsor details, investigational products, recruitment strategies, and any ethical considerations involved in the conduct of the clinical trials.
Fill out your clinical trials ontario conference online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Trials Ontario Conference is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.