Get the free 68GaGa-DOTA-TOC: The First FDA-Approved ...
Show details
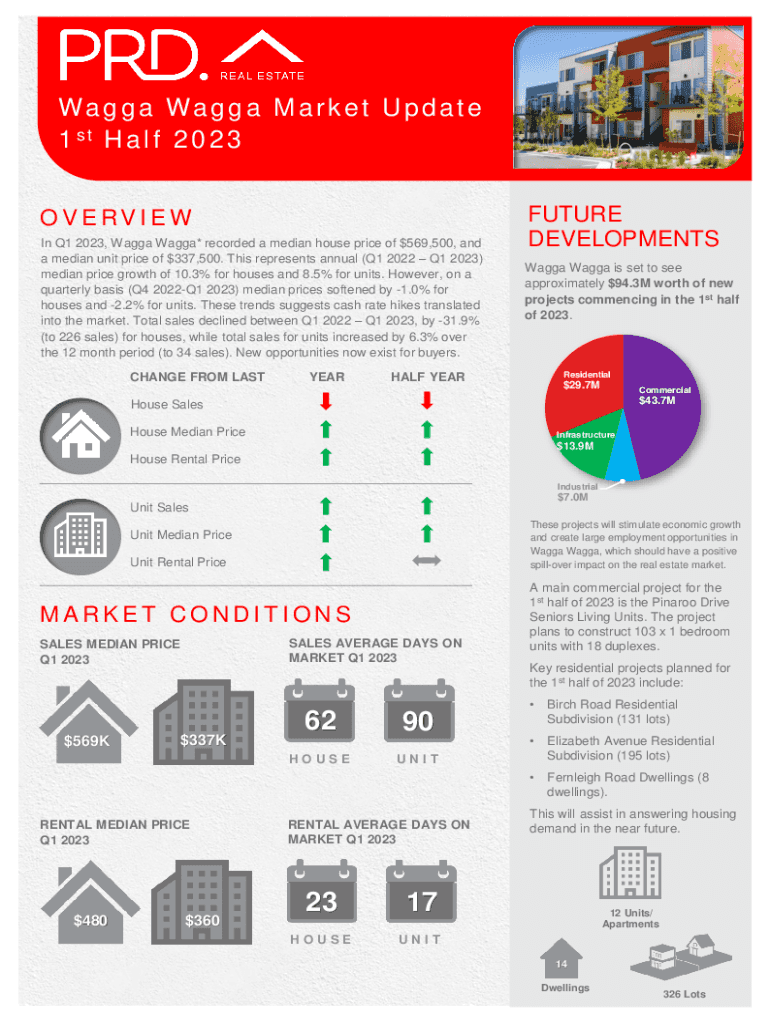
Wa g a Wa g an M an r k e t U p d a t e
1st H an l f 2 0 2 3
OVERVIEW
In Q1 2023, Wagga Wagga* recorded a median house price of $569,500, and
a median unit price of $337,500. This represents annual
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign 68gaga-dota-toc form first fda-approved

Edit your 68gaga-dota-toc form first fda-approved form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your 68gaga-dota-toc form first fda-approved form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit 68gaga-dota-toc form first fda-approved online
To use our professional PDF editor, follow these steps:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit 68gaga-dota-toc form first fda-approved. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out 68gaga-dota-toc form first fda-approved

How to fill out 68gaga-dota-toc form first fda-approved
01
To fill out the 68gaga-dota-toc form first fda-approved, follow these steps:
02
Start by downloading the form from the official FDA website.
03
Read the instructions provided on the form carefully to understand the requirements.
04
Gather all the necessary information and documents that are required to complete the form.
05
Double-check the form to ensure all fields are filled correctly and no information is missing.
06
If there are any supporting documents to be attached, make sure they are properly labeled and attached to the form.
07
Review the completed form once again to verify accuracy and compliance with FDA guidelines.
08
Finally, submit the filled-out form to the designated FDA office by the specified deadline.
09
Keep a copy of the completed form for your records.
Who needs 68gaga-dota-toc form first fda-approved?
01
The 68gaga-dota-toc form first fda-approved is required by individuals or organizations who are involved in the submission and approval process of certain FDA-related activities. This may include pharmaceutical companies, medical device manufacturers, clinical research organizations, or individuals seeking FDA approval for a particular product or service.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my 68gaga-dota-toc form first fda-approved directly from Gmail?
You can use pdfFiller’s add-on for Gmail in order to modify, fill out, and eSign your 68gaga-dota-toc form first fda-approved along with other documents right in your inbox. Find pdfFiller for Gmail in Google Workspace Marketplace. Use time you spend on handling your documents and eSignatures for more important things.
Can I create an electronic signature for the 68gaga-dota-toc form first fda-approved in Chrome?
Yes. With pdfFiller for Chrome, you can eSign documents and utilize the PDF editor all in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a handwritten signature image. You may eSign your 68gaga-dota-toc form first fda-approved in seconds.
Can I edit 68gaga-dota-toc form first fda-approved on an Android device?
Yes, you can. With the pdfFiller mobile app for Android, you can edit, sign, and share 68gaga-dota-toc form first fda-approved on your mobile device from any location; only an internet connection is needed. Get the app and start to streamline your document workflow from anywhere.
What is 68gaga-dota-toc form first fda-approved?
The 68gaga-dota-toc form refers to a specific regulatory filing related to the use of a radioactive drug for medical imaging, which has been approved by the FDA.
Who is required to file 68gaga-dota-toc form first fda-approved?
Entities such as sponsors, manufacturers, or researchers involved in the clinical use of the drug are required to file the 68gaga-dota-toc form.
How to fill out 68gaga-dota-toc form first fda-approved?
Filling out the 68gaga-dota-toc form involves providing specific information about the drug, its intended use, and any relevant clinical data as outlined by the FDA.
What is the purpose of 68gaga-dota-toc form first fda-approved?
The purpose of the 68gaga-dota-toc form is to ensure regulatory compliance and safety monitoring of the radioactive drug used for imaging purposes.
What information must be reported on 68gaga-dota-toc form first fda-approved?
Information that must be reported includes drug composition, dosage, administration routes, clinical trial data, and safety reports.
Fill out your 68gaga-dota-toc form first fda-approved online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

68gaga-Dota-Toc Form First Fda-Approved is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















