Get the free WOMEN'S INTERAGENCY HIV STUDY DRUG FORM 2 - statepi jhsph
Show details
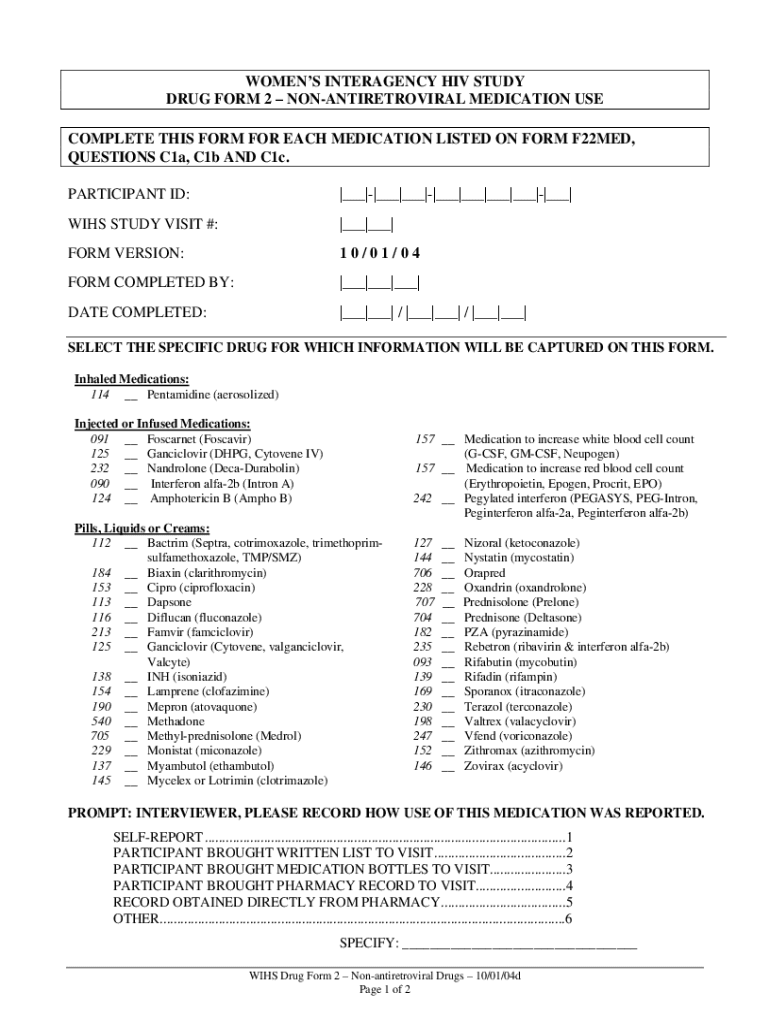
WOMEN INTERAGENCY HIV STUDY
DRUG FORM 2 ANTIRETROVIRAL MEDICATION USE
COMPLETE THIS FORM FOR EACH MEDICATION LISTED ON FORM F22MED,
QUESTIONS C1a, C1b AND C1c.
PARTICIPANT ID:|___||___|___||___|___|___|___||___|WINS
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign womens interagency hiv study

Edit your womens interagency hiv study form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your womens interagency hiv study form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing womens interagency hiv study online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit womens interagency hiv study. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out womens interagency hiv study

How to fill out womens interagency hiv study
01
Start by gathering all the necessary documents and information that will be required to fill out the Women's Interagency HIV Study.
02
Read and understand the instructions provided with the study form.
03
Begin by filling out your personal details, including your name, date of birth, address, and contact information.
04
Provide accurate information about your medical history, including any previous HIV testing and results.
05
Answer all the questions in the form truthfully and to the best of your knowledge.
06
If you are unsure about any question or require assistance, it is recommended to consult with a healthcare professional or contact the study administrators for clarification.
07
Double-check all the information you have entered before submitting the form to ensure accuracy.
08
Follow any additional instructions or guidelines provided by the study for submitting the form or participating in follow-up procedures.
09
Make sure to keep a copy of the filled-out form for your reference.
10
Submit the completed Women's Interagency HIV Study form as per the specified method or to the designated individuals/organizations.
Who needs womens interagency hiv study?
01
The Women's Interagency HIV Study is designed for women who may be at risk of contracting HIV or already living with HIV.
02
It is particularly relevant for women who fall under any of the following categories:
03
- Women engaged in high-risk sexual activities
04
- Women with multiple sexual partners
05
- Women with a history of injecting drug use
06
- Women who are pregnant or planning to become pregnant
07
- Women with a positive HIV diagnosis
08
- Women who are interested in contributing to HIV research and understanding the impact of the virus on women's health
09
Participating in the study can help gather valuable data, contribute to scientific advancements, and improve HIV prevention, diagnosis, and treatment strategies for women.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my womens interagency hiv study directly from Gmail?
pdfFiller’s add-on for Gmail enables you to create, edit, fill out and eSign your womens interagency hiv study and any other documents you receive right in your inbox. Visit Google Workspace Marketplace and install pdfFiller for Gmail. Get rid of time-consuming steps and manage your documents and eSignatures effortlessly.
Can I create an electronic signature for the womens interagency hiv study in Chrome?
Yes. By adding the solution to your Chrome browser, you may use pdfFiller to eSign documents while also enjoying all of the PDF editor's capabilities in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a photo of your handwritten signature using the extension. Whatever option you select, you'll be able to eSign your womens interagency hiv study in seconds.
How do I fill out the womens interagency hiv study form on my smartphone?
Use the pdfFiller mobile app to fill out and sign womens interagency hiv study. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, their features, and how to get started.
What is womens interagency hiv study?
The Women's Interagency HIV Study (WIHS) is a multi-site research project aimed at understanding the natural history of HIV infection and its impact on women's health.
Who is required to file womens interagency hiv study?
Participants in the Women's Interagency HIV Study, which typically includes women living with HIV, are required to file the study.
How to fill out womens interagency hiv study?
To fill out the Women's Interagency HIV Study, participants should follow the specific instructions provided by the research team, which may include completing questionnaires and attending medical examinations.
What is the purpose of womens interagency hiv study?
The purpose of the Women's Interagency HIV Study is to identify the effects of HIV on women's health, understand co-morbidities, and improve treatment and prevention strategies for women.
What information must be reported on womens interagency hiv study?
Participants are typically required to report demographic information, medical history, treatment regimen, and any co-existing health conditions.
Fill out your womens interagency hiv study online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Womens Interagency Hiv Study is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















