Get the free ICH Guidelines for Monitoring Clinical Trial Version
Show details
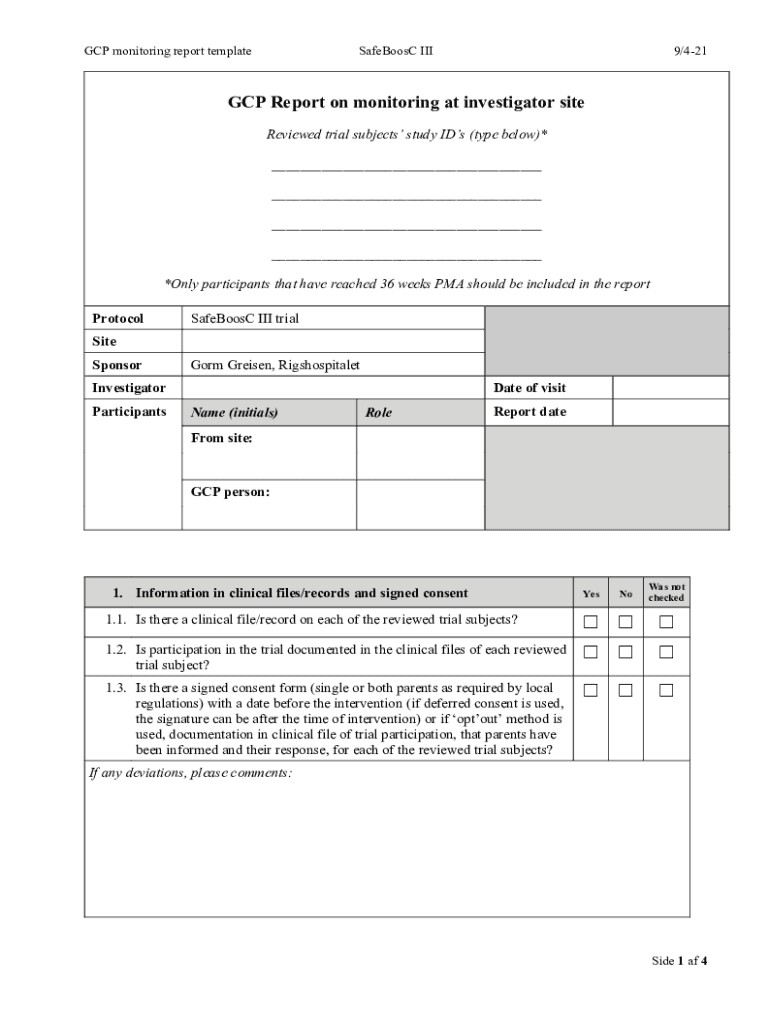
GCP monitoring report templateSafeBoosC III9/421GCPmonitrgepla0SfBsI9/421GCP Report on monitoring at investigator site
Reviewed trial subjects study IDs (type below)*
___
___
___
___
*Only participants
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign ich guidelines for monitoring

Edit your ich guidelines for monitoring form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your ich guidelines for monitoring form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing ich guidelines for monitoring online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit ich guidelines for monitoring. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out ich guidelines for monitoring

How to fill out ich guidelines for monitoring
01
Review the ICH guidelines for monitoring document thoroughly to understand the requirements and recommendations.
02
Determine the scope of the monitoring activities based on the study objectives and regulatory requirements.
03
Identify the specific data to be collected and monitored during the study.
04
Develop a monitoring plan that outlines the frequency, methods, and responsibilities for data collection and review.
05
Train the study staff on the monitoring procedures and ensure that all necessary tools and resources are available.
06
Conduct ongoing monitoring activities according to the plan, including onsite visits, source data verification, and query resolution.
07
Maintain thorough documentation of all monitoring activities and findings.
08
Identify and address any deviations or non-compliance with the guidelines, and take appropriate corrective actions.
09
Regularly review and update the monitoring plan as necessary throughout the duration of the study.
10
Ensure that all monitoring activities are documented and ready for regulatory inspections or audits.
Who needs ich guidelines for monitoring?
01
Clinical research organizations conducting clinical trials.
02
Pharmaceutical companies involved in drug development.
03
Regulatory authorities responsible for reviewing and approving clinical trial data.
04
Investigators and study coordinators involved in conducting clinical trials.
05
Individuals responsible for data management and quality assurance in clinical trials.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send ich guidelines for monitoring for eSignature?
When you're ready to share your ich guidelines for monitoring, you can swiftly email it to others and receive the eSigned document back. You may send your PDF through email, fax, text message, or USPS mail, or you can notarize it online. All of this may be done without ever leaving your account.
How do I complete ich guidelines for monitoring online?
With pdfFiller, you may easily complete and sign ich guidelines for monitoring online. It lets you modify original PDF material, highlight, blackout, erase, and write text anywhere on a page, legally eSign your document, and do a lot more. Create a free account to handle professional papers online.
How do I make edits in ich guidelines for monitoring without leaving Chrome?
Install the pdfFiller Chrome Extension to modify, fill out, and eSign your ich guidelines for monitoring, which you can access right from a Google search page. Fillable documents without leaving Chrome on any internet-connected device.
What is ich guidelines for monitoring?
ICH guidelines for monitoring are a set of standards established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. These guidelines provide a framework for monitoring clinical trials to ensure the safety of participants and the integrity of the data.
Who is required to file ich guidelines for monitoring?
Sponsors of clinical trials, organizations conducting the trials, and regulatory authorities are required to file and adhere to ICH guidelines for monitoring.
How to fill out ich guidelines for monitoring?
To fill out ICH guidelines for monitoring, one must follow the prescribed documentation format, provide relevant data regarding the clinical trial, and ensure that all sections are accurately completed as per the guidelines.
What is the purpose of ich guidelines for monitoring?
The purpose of ICH guidelines for monitoring is to ensure the protection of human rights of trial subjects, the credibility of trial data, and to establish a consistent and compliant approach to monitoring across different regions.
What information must be reported on ich guidelines for monitoring?
Information that must be reported includes protocols for monitoring, reports of adverse events, trial progress updates, participant recruitment statistics, and compliance with regulatory requirements.
Fill out your ich guidelines for monitoring online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Ich Guidelines For Monitoring is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















