Get the free Electron Configurations, Orbital Notation and Quantum ...
Show details
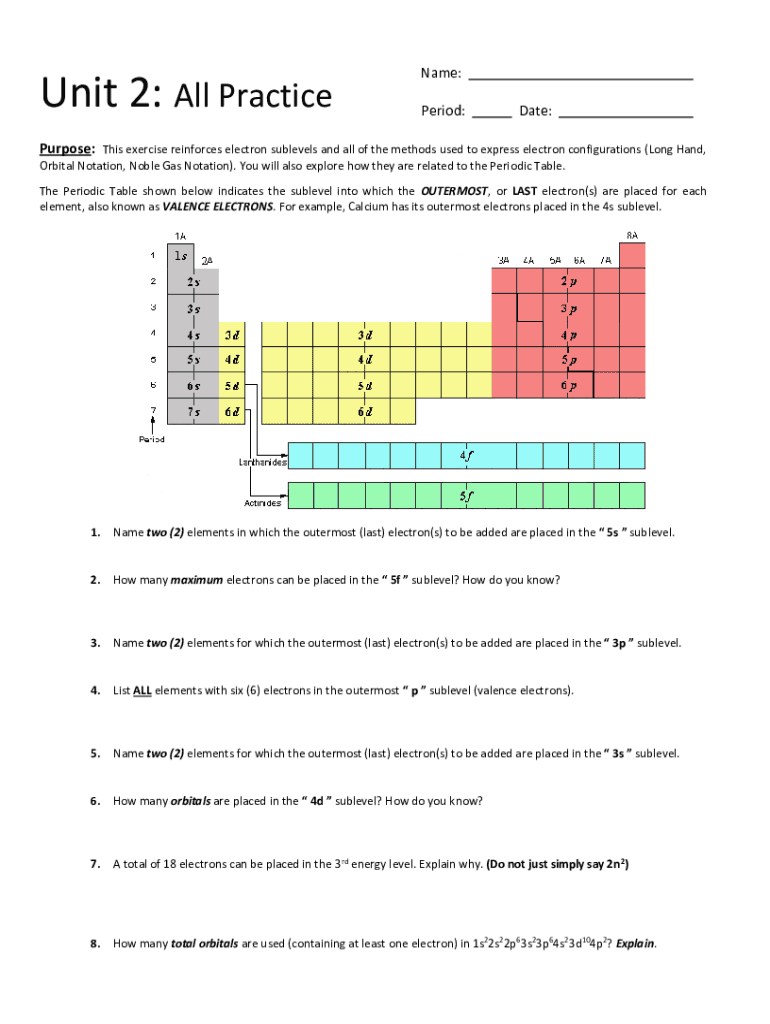
Unit 2: All PracticeName: Period:Date:Purpose: This exercise reinforces electron sublevels and all of the methods used to express electron configurations (Long Hand, Orbital Notation, Noble Gas Notation).
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign electron configurations orbital notation

Edit your electron configurations orbital notation form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your electron configurations orbital notation form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit electron configurations orbital notation online
Use the instructions below to start using our professional PDF editor:
1
Log in to your account. Start Free Trial and sign up a profile if you don't have one yet.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit electron configurations orbital notation. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out electron configurations orbital notation

How to fill out electron configurations orbital notation
01
To fill out electron configurations using orbital notation, follow these steps:
02
Identify the atomic number of the element you are working with.
03
Refer to the periodic table to determine the total number of electrons in the element.
04
Start by placing the electrons in the lowest energy level, which is the first shell (n=1).
05
The first shell can hold a maximum of 2 electrons, so place the first 2 electrons in the 1s orbital.
06
Move on to the second shell (n=2) and fill the orbitals in order of increasing energy: 2s, 2p.
07
- The 2s orbital can hold a maximum of 2 electrons, so place the next 2 electrons in the 2s orbital.
08
- The 2p orbitals can hold a total of 6 electrons (2 electrons per orbital), so place the remaining
09
electrons in the 2p orbitals.
10
Continue filling the remaining shells and orbitals in the same manner, following the order: 3s, 3p, 4s, 3d, 4p,
11
5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
12
Once all the electrons have been placed, the electron configuration is complete.
Who needs electron configurations orbital notation?
01
Electron configurations and orbital notation are needed by chemists, physicists, and students studying chemistry
02
and physics. They help to understand the arrangement of electrons in atoms and provide information about
03
the chemical properties and behavior of elements. Electron configurations are used to determine the
04
periodicity of elements in the periodic table, predict chemical reactions, and explain bonding patterns
05
and molecular structures. They are an essential tool in the field of atomic and molecular physics.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in electron configurations orbital notation?
pdfFiller allows you to edit not only the content of your files, but also the quantity and sequence of the pages. Upload your electron configurations orbital notation to the editor and make adjustments in a matter of seconds. Text in PDFs may be blacked out, typed in, and erased using the editor. You may also include photos, sticky notes, and text boxes, among other things.
How can I edit electron configurations orbital notation on a smartphone?
You can easily do so with pdfFiller's apps for iOS and Android devices, which can be found at the Apple Store and the Google Play Store, respectively. You can use them to fill out PDFs. We have a website where you can get the app, but you can also get it there. When you install the app, log in, and start editing electron configurations orbital notation, you can start right away.
How do I fill out the electron configurations orbital notation form on my smartphone?
The pdfFiller mobile app makes it simple to design and fill out legal paperwork. Complete and sign electron configurations orbital notation and other papers using the app. Visit pdfFiller's website to learn more about the PDF editor's features.
What is electron configurations orbital notation?
Electron configurations orbital notation is a way of representing the arrangement of electrons in an atom's orbitals using a notation that outlines the distribution of electrons among the available energy levels and sublevels.
Who is required to file electron configurations orbital notation?
Electron configurations orbital notation is primarily used in chemistry and physics education and does not involve filing in the legal sense. It is used by students, scientists, and educators to denote electron arrangements in atoms.
How to fill out electron configurations orbital notation?
To fill out electron configurations orbital notation, start by determining the number of electrons in the atom, then fill orbitals according to the Aufbau principle, Hund's rule, and the Pauli exclusion principle, indicating each electron's spin and the occupancy of each orbital.
What is the purpose of electron configurations orbital notation?
The purpose of electron configurations orbital notation is to provide a clear visual representation of how electrons are distributed in an atom, which helps in understanding chemical behavior, bonding, and reactivity.
What information must be reported on electron configurations orbital notation?
In electron configurations orbital notation, the information that must be reported includes the principal quantum number, the type of orbital (s, p, d, f), the number of electrons in each orbital, and their spins.
Fill out your electron configurations orbital notation online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Electron Configurations Orbital Notation is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















