Get the free An Investigator Initiated Study of Monocyte Biomarkers in ...
Show details
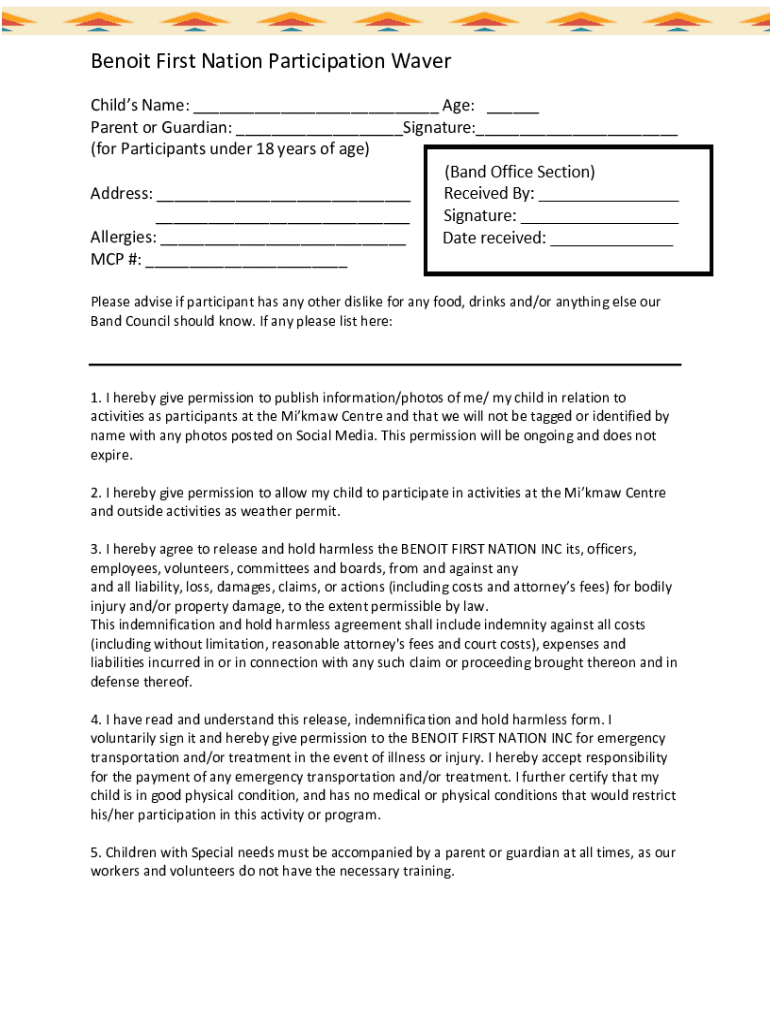
Benoit First Nation Participation Waver Childs Name: ___ Age: ___ Parent or Guardian: ___Signature:___ (for Participants under 18 years of age) Address: ___ ___ Allergies: ___ MCP #: ___ Please advise
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign an investigator initiated study

Edit your an investigator initiated study form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your an investigator initiated study form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing an investigator initiated study online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit an investigator initiated study. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out an investigator initiated study

How to fill out an investigator initiated study
01
Develop a research question or hypothesis that you want to investigate.
02
Identify a study population and determine the sample size needed for statistical significance.
03
Create a study protocol outlining the study design, procedures, and data collection methods.
04
Obtain ethical approval from an Institutional Review Board (IRB) or Research Ethics Committee (REC).
05
Recruit participants for the study and obtain informed consent.
06
Collect and analyze data according to the study protocol.
07
Interpret the results and draw conclusions based on the findings.
08
Prepare a manuscript for publication in a scientific journal.
Who needs an investigator initiated study?
01
Researchers looking to investigate a specific research question or hypothesis.
02
Institutions wanting to generate new knowledge in a particular field of study.
03
Funding agencies seeking to support innovative research projects.
04
Healthcare professionals interested in improving patient care through research.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find an investigator initiated study?
The premium pdfFiller subscription gives you access to over 25M fillable templates that you can download, fill out, print, and sign. The library has state-specific an investigator initiated study and other forms. Find the template you need and change it using powerful tools.
How do I execute an investigator initiated study online?
pdfFiller has made filling out and eSigning an investigator initiated study easy. The solution is equipped with a set of features that enable you to edit and rearrange PDF content, add fillable fields, and eSign the document. Start a free trial to explore all the capabilities of pdfFiller, the ultimate document editing solution.
How do I make edits in an investigator initiated study without leaving Chrome?
Add pdfFiller Google Chrome Extension to your web browser to start editing an investigator initiated study and other documents directly from a Google search page. The service allows you to make changes in your documents when viewing them in Chrome. Create fillable documents and edit existing PDFs from any internet-connected device with pdfFiller.
What is an investigator initiated study?
An investigator initiated study is a research project that is designed, initiated, and managed by a clinician or researcher, rather than a pharmaceutical company or other sponsor. The investigator is responsible for the study's design, methodology, and conduct.
Who is required to file an investigator initiated study?
Researchers and clinicians who intend to conduct an investigator initiated study must file the study, often through their institution’s regulatory or ethics board, to ensure adherence to ethical standards and regulations.
How to fill out an investigator initiated study?
To fill out an investigator initiated study, the investigator should complete a study protocol, which includes details about the study design, objectives, methodology, and participant recruitment. The protocol must then be submitted for ethical review and approval.
What is the purpose of an investigator initiated study?
The purpose of an investigator initiated study is to explore clinical hypotheses, advance medical knowledge, and evaluate new treatments or interventions from a researcher’s perspective, often addressing questions not covered by industry-sponsored trials.
What information must be reported on an investigator initiated study?
Information that must be reported on an investigator initiated study includes study objectives, design, methodology, statistical analysis plans, enrollment numbers, safety data, and outcomes. Adverse events and other significant findings during the study must also be documented.
Fill out your an investigator initiated study online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

An Investigator Initiated Study is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.

















