Get the free Samples Management and Data Protection through SARS ...
Show details
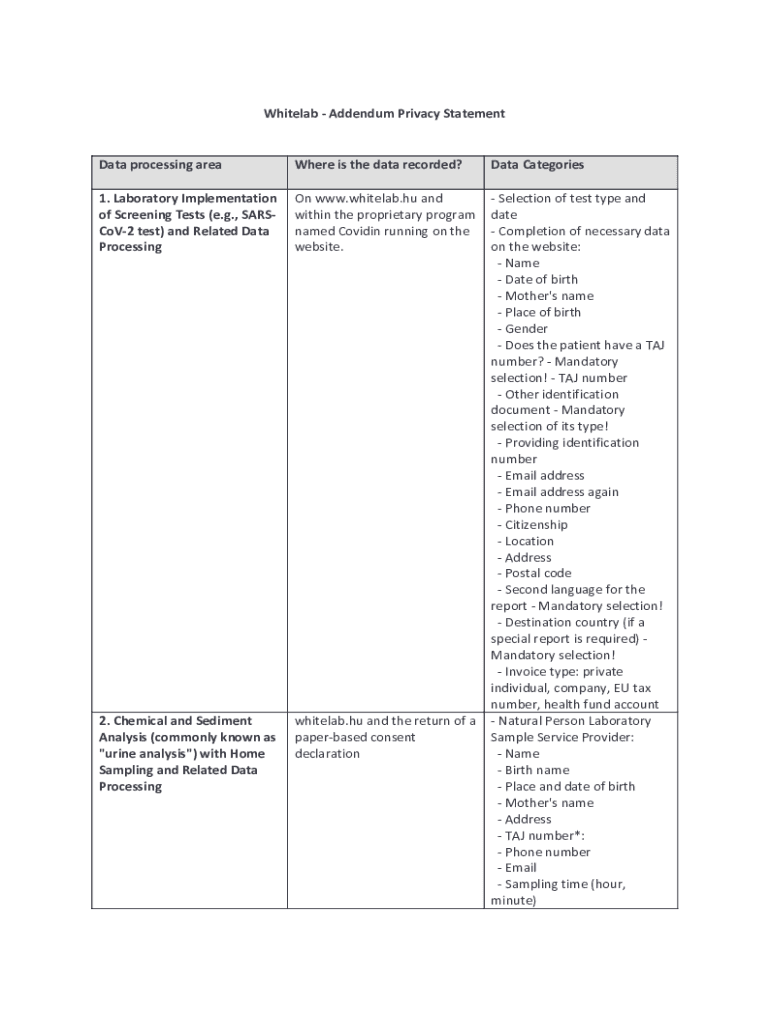
Whitelab Addendum Privacy Statement Data processing areaWhere is the data recorded?Data Categories1. Laboratory Implementation of Screening Tests (e.g., SARSCoV2 test) and Related Data ProcessingOn
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign samples management and data

Edit your samples management and data form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your samples management and data form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing samples management and data online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit samples management and data. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out samples management and data

How to fill out samples management and data
01
Identify the purpose of collecting samples and data
02
Develop a clear and organized system for labeling and storing samples
03
Create a data management plan including data entry procedures and storage
04
Train staff on proper procedures for collecting, handling, and entering samples and data
05
Regularly review and update the samples management and data system for accuracy and efficiency
Who needs samples management and data?
01
Research institutions and laboratories
02
Biotechnology and pharmaceutical companies
03
Environmental agencies and organizations
04
Healthcare facilities and diagnostic laboratories
05
Academic institutions conducting scientific studies
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send samples management and data for eSignature?
samples management and data is ready when you're ready to send it out. With pdfFiller, you can send it out securely and get signatures in just a few clicks. PDFs can be sent to you by email, text message, fax, USPS mail, or notarized on your account. You can do this right from your account. Become a member right now and try it out for yourself!
Where do I find samples management and data?
The premium pdfFiller subscription gives you access to over 25M fillable templates that you can download, fill out, print, and sign. The library has state-specific samples management and data and other forms. Find the template you need and change it using powerful tools.
How can I fill out samples management and data on an iOS device?
Make sure you get and install the pdfFiller iOS app. Next, open the app and log in or set up an account to use all of the solution's editing tools. If you want to open your samples management and data, you can upload it from your device or cloud storage, or you can type the document's URL into the box on the right. After you fill in all of the required fields in the document and eSign it, if that is required, you can save or share it with other people.
What is samples management and data?
Samples management and data refers to the systematic process of tracking, organizing, and maintaining records of samples collected, tested, or analyzed for various purposes, including regulatory compliance and quality assurance.
Who is required to file samples management and data?
Entities that collect, analyze, or distribute samples for testing or regulatory purposes, such as laboratories, manufacturers, and distributors, are typically required to file samples management and data.
How to fill out samples management and data?
To fill out samples management and data, one should gather relevant information about each sample, including its identification, collection date, analysis results, and any applicable regulatory details, and enter this data into the designated reporting format or system.
What is the purpose of samples management and data?
The purpose of samples management and data is to ensure proper tracking, compliance with regulations, facilitate quality control, and support decision-making processes regarding the use and safety of samples.
What information must be reported on samples management and data?
Information that must be reported typically includes sample identification details, collection dates, testing results, storage conditions, chain of custody information, and any pertinent compliance data.
Fill out your samples management and data online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Samples Management And Data is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















