Get the free A Nested Case-Control Study of Allopregnanolone and ...
Show details
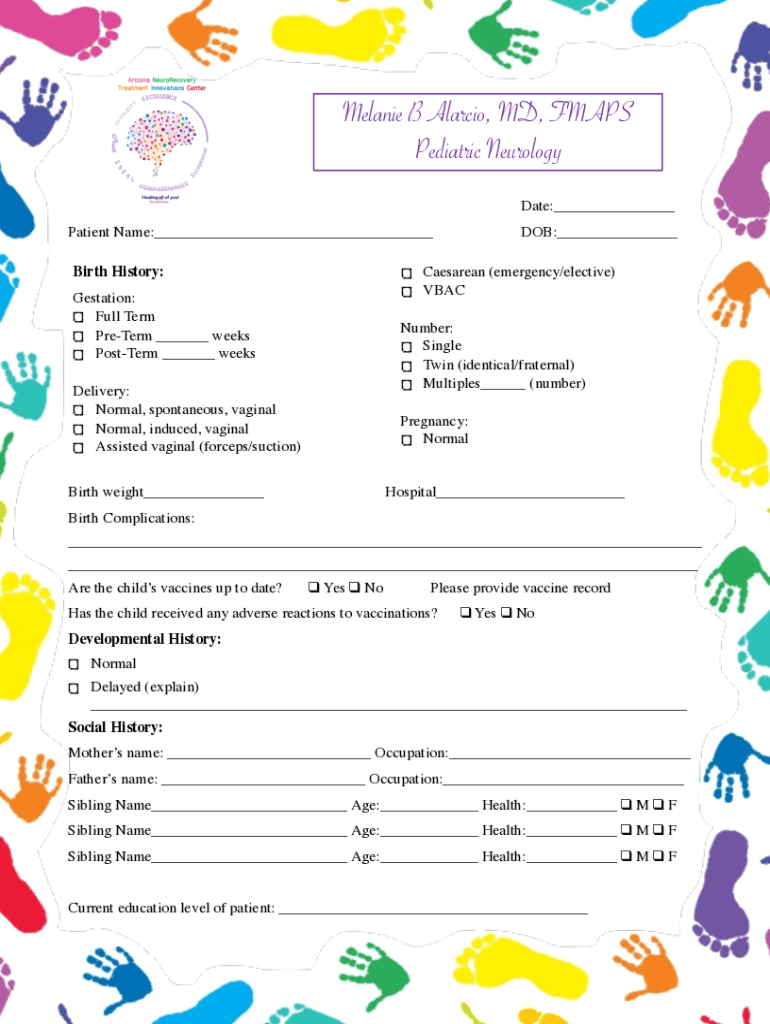
Melanie B Alarcio, MD, FMAPS Pediatric Neurology Date:___ Patient Name:___ Birth History: Gestation: Full Term PreTerm ___ weeks PostTerm ___ weeks Delivery: Normal, spontaneous, vaginal Normal, induced,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign a nested case-control study

Edit your a nested case-control study form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your a nested case-control study form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing a nested case-control study online
Use the instructions below to start using our professional PDF editor:
1
Sign into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit a nested case-control study. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out a nested case-control study

How to fill out a nested case-control study
01
Identify a well-defined cohort of individuals who are free of the outcome of interest at the beginning of the study.
02
Collect data on potential risk factors for the outcome of interest from this cohort.
03
Identify cases that develop the outcome of interest within the cohort during the study period.
04
Select a sample of individuals from the original cohort who did not develop the outcome of interest to serve as controls.
05
Analyze the data to investigate the association between the risk factors and the outcome of interest.
Who needs a nested case-control study?
01
Researchers looking to investigate the etiology of a specific disease or outcome.
02
Individuals interested in studying risk factors for a particular condition within a well-defined population.
03
Health professionals aiming to understand the relationship between exposures and disease outcomes.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete a nested case-control study online?
Completing and signing a nested case-control study online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
How do I make changes in a nested case-control study?
The editing procedure is simple with pdfFiller. Open your a nested case-control study in the editor. You may also add photos, draw arrows and lines, insert sticky notes and text boxes, and more.
How do I edit a nested case-control study in Chrome?
Install the pdfFiller Google Chrome Extension to edit a nested case-control study and other documents straight from Google search results. When reading documents in Chrome, you may edit them. Create fillable PDFs and update existing PDFs using pdfFiller.
What is a nested case-control study?
A nested case-control study is a type of observational study design where cases of a particular outcome are identified from a cohort, and controls are selected from the same cohort, allowing researchers to investigate the associations between exposures and outcomes in a more efficient manner.
Who is required to file a nested case-control study?
Typically, researchers or organizations conducting the study are required to file a nested case-control study, particularly if it involves data collection or analysis related to public health or requires ethical oversight.
How to fill out a nested case-control study?
To fill out a nested case-control study, researchers need to clearly define the population, identify cases and controls, collect exposure data, and document the analysis plan and results in a structured format.
What is the purpose of a nested case-control study?
The purpose of a nested case-control study is to efficiently evaluate the relationship between a potential risk factor and a specific outcome within a defined cohort, thus helping to avoid the biases and resource intensiveness of larger case-control studies.
What information must be reported on a nested case-control study?
Information that must be reported includes the study population, sampling method for cases and controls, definitions for exposure and outcome, methodology used for data collection and analysis, and results including any statistical comparisons.
Fill out your a nested case-control study online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

A Nested Case-Control Study is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.