PVDOMICS Study Form 187 2023-2025 free printable template
Show details
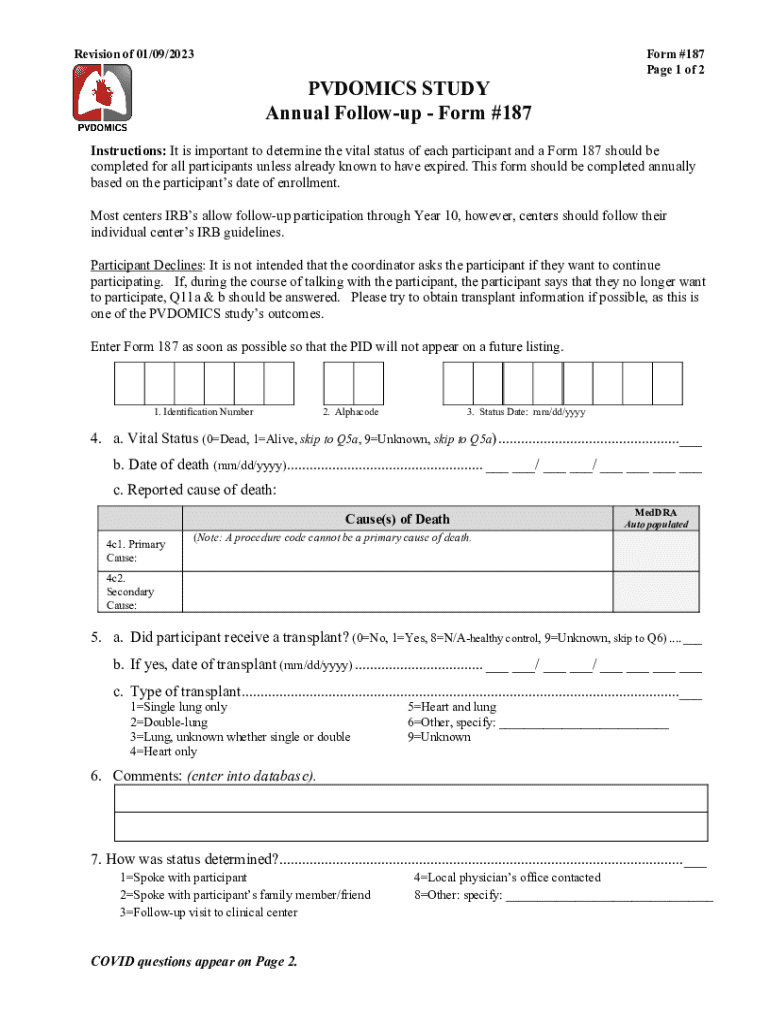
Revision of 01/09/2023PVDOMICS STUDY
Annual Followup Form #187Form #187
Page 1 of 2Instructions: It is important to determine the vital status of each participant and a Form 187 should be
completed
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign pvdomics study annual follow-up

Edit your pvdomics study annual follow-up form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your pvdomics study annual follow-up form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing pvdomics study annual follow-up online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit pvdomics study annual follow-up. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
PVDOMICS Study Form 187 Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out pvdomics study annual follow-up

How to fill out PVDOMICS Study Form 187
01
Begin by entering your personal details such as name, date of birth, and contact information in the designated fields.
02
Proceed to the health history section and provide information about any previous medical conditions, surgeries, or treatments.
03
Fill out the section related to current medications by listing all medications you are taking, including dosage and frequency.
04
Complete the lifestyle questionnaire by answering questions regarding your diet, exercise habits, and tobacco or alcohol use.
05
Review the demographics section and provide information regarding your ethnicity, employment status, and education level.
06
Ensure all required signatures and dates are filled out at the end of the form to validate your submission.
07
Double-check all entries for accuracy before submitting the form to avoid delays.
Who needs PVDOMICS Study Form 187?
01
PVDOMICS Study Form 187 is needed by individuals participating in the PVDOMICS study, healthcare professionals involved in patient data collection, and researchers analyzing the data for study outcomes.
Fill
form
: Try Risk Free






People Also Ask about
How much does the CEO of the Cleveland Clinic make?
Edmund Sabanegh, president, Cleveland Clinic Main Campus and Regional Hospitals - $1,087,264, and $45,938 in other compensation.
How much does Cleveland Clinic profit?
Total unrestricted revenues increased 4.5 percent year over year to $13 billion. This is largely attributable to net patient service revenue, which was $11.6 billion in 2022 and $10.9 billion in 2021.
How do I contact the Cleveland Clinic PSLF?
Call Cleveland Clinic We're here to answer your questions and help you find the best care. You can reach us toll-free at 800.223. 2273.
How much does Cleveland Clinic make a year?
Cleveland Clinic grew 2022 revenue roughly 5% to $13 billion from the prior year, but didn't outpace expenses as costs increased nearly 14% to $12.4 billion before interest, depreciation and amortization. Investment income helped pull the Midwest provider into the red as non-operating losses totaled $1 billion.
Do you need a referral to be seen at Cleveland Clinic?
Cleveland Clinic does not require a referral, however your insurance company might require one to provide coverage for your visit or procedure. For more details, please review our billing checklist.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send pvdomics study annual follow-up for eSignature?
pvdomics study annual follow-up is ready when you're ready to send it out. With pdfFiller, you can send it out securely and get signatures in just a few clicks. PDFs can be sent to you by email, text message, fax, USPS mail, or notarized on your account. You can do this right from your account. Become a member right now and try it out for yourself!
How do I execute pvdomics study annual follow-up online?
With pdfFiller, you may easily complete and sign pvdomics study annual follow-up online. It lets you modify original PDF material, highlight, blackout, erase, and write text anywhere on a page, legally eSign your document, and do a lot more. Create a free account to handle professional papers online.
How do I make edits in pvdomics study annual follow-up without leaving Chrome?
Download and install the pdfFiller Google Chrome Extension to your browser to edit, fill out, and eSign your pvdomics study annual follow-up, which you can open in the editor with a single click from a Google search page. Fillable documents may be executed from any internet-connected device without leaving Chrome.
What is PVDOMICS Study Form 187?
PVDOMICS Study Form 187 is a research document used to collect data related to patients with peripheral vascular disease as part of the PVDOMICS initiative.
Who is required to file PVDOMICS Study Form 187?
Healthcare professionals involved in the PVDOMICS study, including clinicians and researchers responsible for patient data collection, are required to file PVDOMICS Study Form 187.
How to fill out PVDOMICS Study Form 187?
To fill out PVDOMICS Study Form 187, participants should follow the specific guidelines provided in the study protocol, ensuring accurate and complete reporting of patient data.
What is the purpose of PVDOMICS Study Form 187?
The purpose of PVDOMICS Study Form 187 is to standardize the data collection process for patients with peripheral vascular disease to facilitate research and improve patient outcomes.
What information must be reported on PVDOMICS Study Form 187?
Information that must be reported on PVDOMICS Study Form 187 includes patient demographics, clinical history, diagnostic results, and treatment details related to peripheral vascular disease.
Fill out your pvdomics study annual follow-up online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Pvdomics Study Annual Follow-Up is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.






















