Get the free Integrated post-approval application form for amendments, renewals ...
Show details
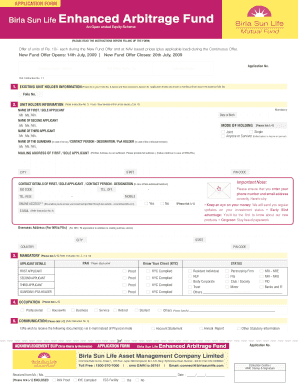
Fraser Health Research Ethics Board Department of Evaluation and Research Services #400, Central City Tower, Surrey, BC V3T 0H1 Phone: 604.587.4436 Fax: 604.930-5425 INTEGRATED POST-APPROVAL APPLICATION
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign integrated post-approval application form

Edit your integrated post-approval application form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your integrated post-approval application form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit integrated post-approval application form online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit integrated post-approval application form. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out integrated post-approval application form

How to Fill Out Integrated Post-Approval Application Form:
01
Start by carefully reading the instructions and guidelines provided with the application form. This will ensure that you have a clear understanding of the requirements and can fill out the form accurately.
02
Begin with the basic information section of the form, which typically asks for details such as the applicant's name, contact information, and any relevant identification numbers or codes.
03
Proceed to the specific sections of the form that pertain to the post-approval application. This may include providing information about the original approval, such as the approval number, date, and any conditions or restrictions that were previously imposed.
04
Clearly state the purpose of the post-approval application. Whether it is for a modification, extension, renewal, or any other post-approval request, clearly articulate the reasons and objectives.
05
Provide a detailed description of the proposed changes or modifications that are being sought through the post-approval application. This may include information about the new or modified processes, procedures, ingredients, formulations, or any other relevant factors.
06
Include any supporting documents or evidence that are required to substantiate the post-approval application. This may include scientific data, research findings, clinical trial results, safety assessments, or any other relevant documentation.
07
If there are any specific fees or payments associated with the post-approval application, ensure that they are included as per the instructions provided. This may require attaching a payment method or providing necessary financial information.
08
Finally, carefully review the completed application form to ensure accuracy and completeness. Double-check all the provided information, signatures, and attachments before submitting it according to the specified method (online submission, mail, etc.).
Who Needs Integrated Post-Approval Application Form:
01
Pharmaceutical companies or manufacturers who have previously obtained approval for their products and now seek to make changes, modifications, or updates to the approved processes, formulations, labeling, or other aspects.
02
Researchers or scientists involved in clinical trials or medical studies who need to seek post-approval authorization for further research, expanded indications, or modifications to their existing protocols.
03
Regulatory bodies or authorities responsible for overseeing the approval and regulation of drugs, medical devices, or other regulated products that require an application process to assess the post-approval requests. These bodies may include government agencies, health authorities, or specialized regulatory bodies.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is integrated post-approval application form?
Integrated post-approval application form is a form submitted to request changes to an approved product.
Who is required to file integrated post-approval application form?
The holder of the marketing authorization is required to file the integrated post-approval application form.
How to fill out integrated post-approval application form?
The form should be completed following the specific instructions provided by the regulatory agency.
What is the purpose of integrated post-approval application form?
The purpose of the form is to document and request changes to an approved product.
What information must be reported on integrated post-approval application form?
The form must include detailed information about the proposed changes to the product.
How do I make changes in integrated post-approval application form?
The editing procedure is simple with pdfFiller. Open your integrated post-approval application form in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I edit integrated post-approval application form in Chrome?
Adding the pdfFiller Google Chrome Extension to your web browser will allow you to start editing integrated post-approval application form and other documents right away when you search for them on a Google page. People who use Chrome can use the service to make changes to their files while they are on the Chrome browser. pdfFiller lets you make fillable documents and make changes to existing PDFs from any internet-connected device.
Can I sign the integrated post-approval application form electronically in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your integrated post-approval application form.
Fill out your integrated post-approval application form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Integrated Post-Approval Application Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.