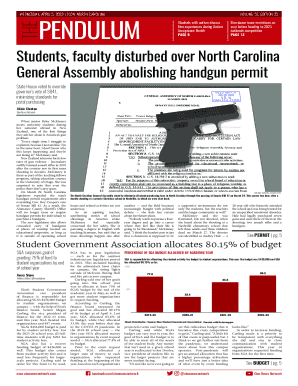
Get the free Draft Wireframes for FDA Form 3965 (SE)
Show details
An ofcial website of the United States government Heres how you knowWelcome to CTP Portal NextGen The U.S. Food and Drug Administration\'s (FDA), Center for Tobacco Products (CTP) developed the CTP
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign draft wireframes for fda

Edit your draft wireframes for fda form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your draft wireframes for fda form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing draft wireframes for fda online
To use our professional PDF editor, follow these steps:
1
Sign into your account. It's time to start your free trial.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit draft wireframes for fda. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out draft wireframes for fda

How to fill out draft wireframes for fda
01
Review FDA guidelines to understand the wireframe requirements.
02
Identify the purpose of the product and the specific use cases.
03
Gather all necessary requirements and stakeholder inputs.
04
Outline the main features and functionalities needed for the product.
05
Select appropriate wireframing tools (e.g., Sketch, Axure, Figma).
06
Start creating low-fidelity wireframes to visualize layout and structure.
07
Include labels for interactive elements and notes explaining each section.
08
Iterate on wireframes based on feedback from stakeholders.
09
Create high-fidelity wireframes if detailed design input is necessary.
10
Ensure all wireframes are clearly documented and easily accessible.
Who needs draft wireframes for fda?
01
Product developers who are creating medical devices or software for FDA approval.
02
Design teams working on user interfaces for healthcare products.
03
Regulatory affairs professionals who need to ensure compliance with FDA requirements.
04
Stakeholders involved in the development process for user experience validation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make edits in draft wireframes for fda without leaving Chrome?
Install the pdfFiller Google Chrome Extension in your web browser to begin editing draft wireframes for fda and other documents right from a Google search page. When you examine your documents in Chrome, you may make changes to them. With pdfFiller, you can create fillable documents and update existing PDFs from any internet-connected device.
How do I edit draft wireframes for fda on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share draft wireframes for fda from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
How do I fill out draft wireframes for fda on an Android device?
Complete draft wireframes for fda and other documents on your Android device with the pdfFiller app. The software allows you to modify information, eSign, annotate, and share files. You may view your papers from anywhere with an internet connection.
What is draft wireframes for fda?
Draft wireframes for the FDA are preliminary layouts or blueprints that outline the structure and design of a medical device, application, or study submission intended for review by the FDA.
Who is required to file draft wireframes for fda?
Any organization or individual that is developing a medical device, software, or application that requires FDA approval must file draft wireframes.
How to fill out draft wireframes for fda?
To fill out draft wireframes for the FDA, developers should include detailed depictions of each screen or component, annotations explaining functionality, and ensure compliance with FDA guidelines.
What is the purpose of draft wireframes for fda?
The purpose of draft wireframes for the FDA is to provide a visual representation of the product's layout and functionality, aiding in the review and approval process.
What information must be reported on draft wireframes for fda?
Draft wireframes must report information such as screen layouts, user interfaces, navigation flows, and associated functionalities of the device or application.
Fill out your draft wireframes for fda online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Draft Wireframes For Fda is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.