Get the free Form Fda 483
Show details
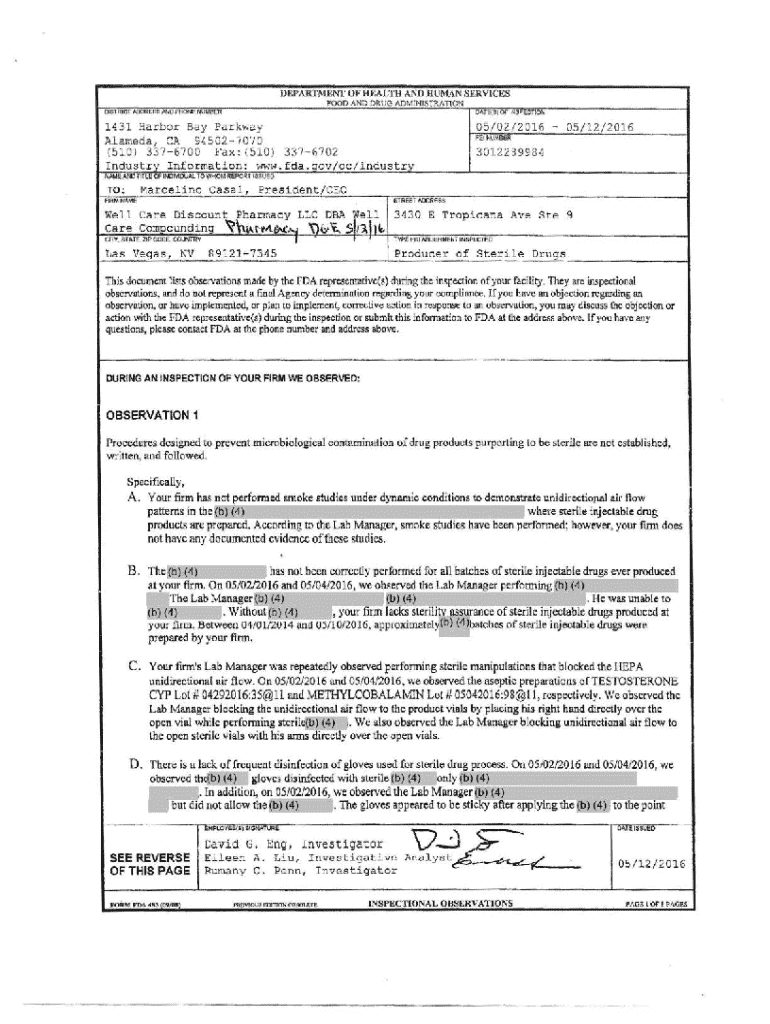
This document outlines the inspectional observations made by the FDA during the inspection of Well Care Discount Pharmacy LLC, focusing on multiple deficiencies related to sterile drug production processes and compliance with regulatory standards.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign form fda 483

Edit your form fda 483 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your form fda 483 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing form fda 483 online
Follow the steps below to use a professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit form fda 483. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out form fda 483

How to fill out form fda 483
01
Obtain a copy of Form FDA 483 from the FDA website or your local FDA office.
02
Identify the establishment name, address, and date of the inspection in the top section of the form.
03
Clearly describe each observation made during the inspection in the appropriate sections, ensuring to specify the area and the nature of the violation.
04
Use concise language and document the observations in chronological order.
05
Provide any supporting documentation or evidence alongside the observations, if applicable.
06
Include any responses or corrective actions proposed by the facility in response to the observations.
07
Review the completed form for clarity and accuracy before submission.
08
Sign and date the form, indicating the responsible individual if required.
Who needs form fda 483?
01
Form FDA 483 is required by establishments inspected by the FDA that are found to be in violation of regulatory standards, including manufacturers of pharmaceuticals, medical devices, and food products.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify form fda 483 without leaving Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your form fda 483 into a dynamic fillable form that can be managed and signed using any internet-connected device.
Can I create an electronic signature for signing my form fda 483 in Gmail?
Use pdfFiller's Gmail add-on to upload, type, or draw a signature. Your form fda 483 and other papers may be signed using pdfFiller. Register for a free account to preserve signed papers and signatures.
How do I complete form fda 483 on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your form fda 483 from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is form fda 483?
Form FDA 483 is a form issued by the U.S. Food and Drug Administration (FDA) to indicate that an inspection has revealed conditions that may constitute violations of the Food Drug and Cosmetic (FD&C) Act.
Who is required to file form fda 483?
Form FDA 483 is not filed by individuals, but rather issued to manufacturers, processors, and packers of FDA-regulated products who are found to have issues during an FDA inspection.
How to fill out form fda 483?
Form FDA 483 is filled out by FDA inspectors during an inspection. They document observations of non-compliance regarding the FD&C Act. The observations are then shared with the inspected facility.
What is the purpose of form fda 483?
The purpose of Form FDA 483 is to communicate to the management of a company the observations made during an inspection that indicate potential regulatory violations.
What information must be reported on form fda 483?
Form FDA 483 must report specific observations related to violations or concerns found during the inspection, detailing the nature of the issues and referencing relevant regulations.
Fill out your form fda 483 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Form Fda 483 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















