Get the free Specimen Labeling Event Investigation Tool
Show details
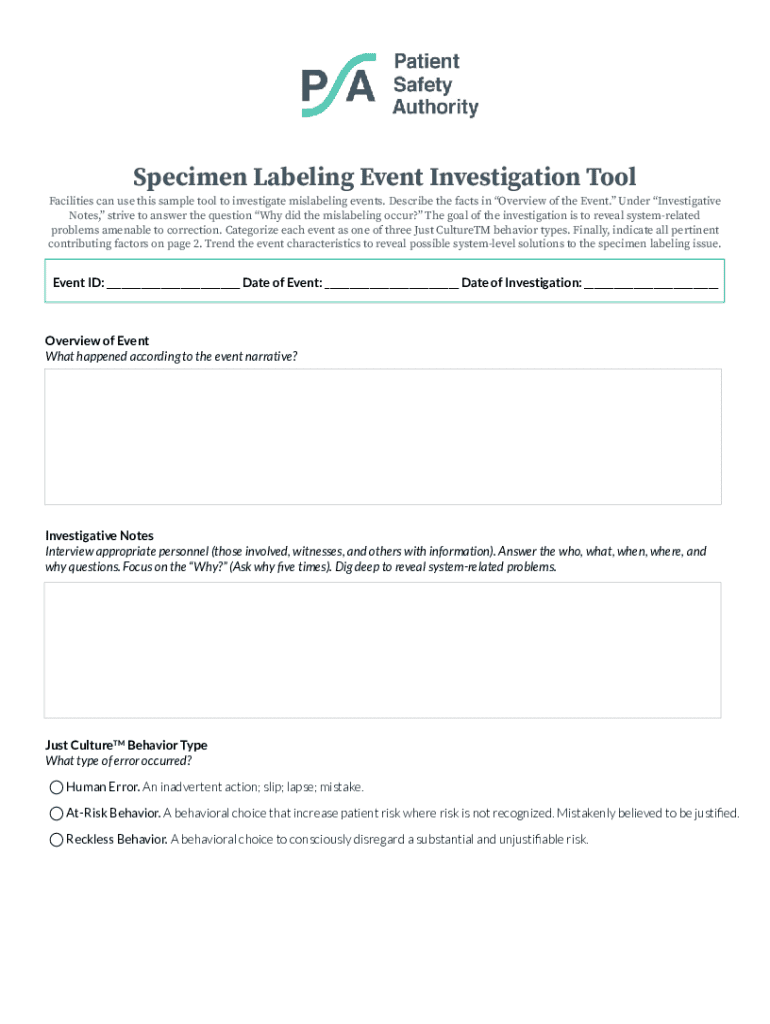
Facilities can use this sample tool to investigate mislabeling events by providing an overview, gathering investigative notes, categorizing errors, and identifying contributing factors to reveal system-related problems.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign specimen labeling event investigation

Edit your specimen labeling event investigation form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your specimen labeling event investigation form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit specimen labeling event investigation online
Follow the steps down below to benefit from the PDF editor's expertise:
1
Log in to your account. Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit specimen labeling event investigation. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
Dealing with documents is simple using pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out specimen labeling event investigation

How to fill out specimen labeling event investigation
01
Gather all necessary information about the specimen, including patient details and sample type.
02
Clearly label each specimen with unique identifiers, such as barcode or ID number.
03
Document the collection date and time, as well as the person collecting the specimen.
04
Ensure proper handling and storage conditions are noted prior to shipping or analysis.
05
Record any relevant observations or incidents that occurred during the specimen collection process.
06
Review the completed labeling and investigation forms for accuracy before submission.
Who needs specimen labeling event investigation?
01
Laboratory personnel handling specimens.
02
Healthcare providers involved in patient treatment.
03
Quality assurance teams ensuring compliance with labeling standards.
04
Regulatory agencies overseeing laboratory practices.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit specimen labeling event investigation from Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including specimen labeling event investigation, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
Can I create an eSignature for the specimen labeling event investigation in Gmail?
Create your eSignature using pdfFiller and then eSign your specimen labeling event investigation immediately from your email with pdfFiller's Gmail add-on. To keep your signatures and signed papers, you must create an account.
How do I complete specimen labeling event investigation on an Android device?
On Android, use the pdfFiller mobile app to finish your specimen labeling event investigation. Adding, editing, deleting text, signing, annotating, and more are all available with the app. All you need is a smartphone and internet.
What is specimen labeling event investigation?
Specimen labeling event investigation is a process to investigate discrepancies or issues related to the labeling of biological specimens, ensuring that they are accurately identified and tracked throughout the testing process.
Who is required to file specimen labeling event investigation?
Individuals or organizations involved in the handling, processing, and testing of biological specimens, such as laboratories and healthcare providers, are required to file specimen labeling event investigations when discrepancies occur.
How to fill out specimen labeling event investigation?
To fill out a specimen labeling event investigation, one should provide detailed information about the specimen in question, including the type of specimen, the nature of the labeling issue, the individuals involved, the timeline of events, and any corrective actions taken.
What is the purpose of specimen labeling event investigation?
The purpose of a specimen labeling event investigation is to ensure the integrity and accuracy of specimen identification, reduce the risk of errors in testing and reporting, and maintain patient safety.
What information must be reported on specimen labeling event investigation?
The information that must be reported includes the specimen type, details of the labeling discrepancy, the individuals involved, timestamps of the event, potential impacts on test results, and steps taken to resolve the issue.
Fill out your specimen labeling event investigation online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Specimen Labeling Event Investigation is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.

















