Get the free A Phase I Dose Escalation Study of Harmine in Healthy Subjects
Show details
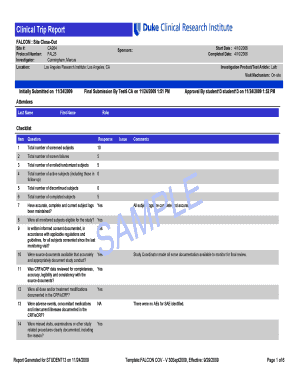
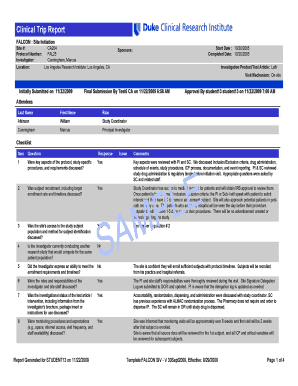
A Phase I Dose Escalation Study of Harmine in Healthy Subjects PI: James Murrough, MD, PhD NCT05526430 Document Date: June 6, 2023THE MOUNT SINAI HEALTH SYSTEM CONSENT FORM TO VOLUNTEER IN A RESEARCH
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign a phase i dose

Edit your a phase i dose form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your a phase i dose form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing a phase i dose online
Follow the guidelines below to use a professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit a phase i dose. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out a phase i dose

How to fill out a phase i dose
01
Prepare all necessary materials and documentation required for the phase I dose.
02
Review the protocol and inclusion/exclusion criteria for patient eligibility.
03
Calculate the appropriate dose based on the study design and patient characteristics.
04
Obtain informed consent from the patient participating in the study.
05
Administer the phase I dose as per the safety guidelines and monitoring requirements.
06
Record all administration details and monitor the patient for any adverse reactions.
Who needs a phase i dose?
01
Individuals participating in clinical trials that are investigating new medications or treatments.
02
Patients who meet the specific inclusion criteria set forth in the study protocol.
03
Healthy volunteers or patients with targeted conditions, depending on the study focus.
04
Regulatory bodies and researchers who require dosing information for trial initiation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find a phase i dose?
It’s easy with pdfFiller, a comprehensive online solution for professional document management. Access our extensive library of online forms (over 25M fillable forms are available) and locate the a phase i dose in a matter of seconds. Open it right away and start customizing it using advanced editing features.
How do I edit a phase i dose in Chrome?
Install the pdfFiller Google Chrome Extension to edit a phase i dose and other documents straight from Google search results. When reading documents in Chrome, you may edit them. Create fillable PDFs and update existing PDFs using pdfFiller.
How do I edit a phase i dose on an iOS device?
Yes, you can. With the pdfFiller mobile app, you can instantly edit, share, and sign a phase i dose on your iOS device. Get it at the Apple Store and install it in seconds. The application is free, but you will have to create an account to purchase a subscription or activate a free trial.
What is a phase i dose?
A Phase I dose refers to the initial amount of a drug administered in the first stage of clinical trials, primarily to assess safety, tolerability, and pharmacokinetics in a small group of healthy volunteers or patients.
Who is required to file a phase i dose?
Researchers, pharmaceutical companies, or any organization conducting clinical trials on new drugs are required to file a Phase I dose with regulatory authorities to obtain approval before starting the trial.
How to fill out a phase i dose?
To fill out a Phase I dose, investigators must provide detailed information including the drug formulation, dose levels, administration route, study design, eligibility criteria, and informed consent procedures.
What is the purpose of a phase i dose?
The purpose of a Phase I dose is to evaluate the safety and tolerability of a drug in humans, establish dosing parameters for future phases, and identify any potential side effects or adverse reactions.
What information must be reported on a phase i dose?
Information that must be reported includes the drug's chemical properties, proposed dosage, patient selection criteria, monitoring procedures, safety assessments, and potential risks associated with the treatment.
Fill out your a phase i dose online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

A Phase I Dose is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.