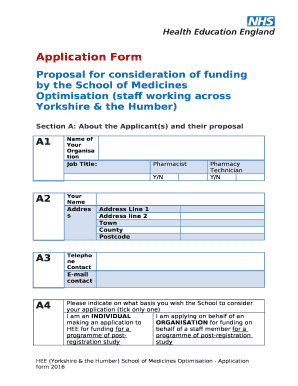
Get the free CUMC IRB# AAAQ3153 - Version Date: 05SEP2018
Show details
Columbia University Medical Center Herbert Irving Comprehensive Cancer Center CUMC IRB# AAAQ3153 Version Date: 05SEP2018NCT02716038CUMC IRB#: AAAQ3153 Celgene Protocol Identifier AXCLNSCLCPI005652
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign cumc irb aaaq3153

Edit your cumc irb aaaq3153 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your cumc irb aaaq3153 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit cumc irb aaaq3153 online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit cumc irb aaaq3153. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out cumc irb aaaq3153

How to fill out cumc irb aaaq3153
01
Visit the CUMC IRB website and locate the AAQ3153 form.
02
Carefully read the instructions provided for completing the form.
03
Fill in the project title and principal investigator's details.
04
Provide a summary of the research project, including objectives and methods.
05
Describe the potential risks to participants and how they will be mitigated.
06
Include information about participant recruitment and informed consent procedures.
07
Attach any supporting documents required, such as questionnaires or consent forms.
08
Review the completed form for accuracy and completeness.
09
Submit the form electronically via the designated submission portal.
Who needs cumc irb aaaq3153?
01
Researchers planning to conduct studies involving human participants at CUMC.
02
Students conducting research requiring IRB approval for their thesis or dissertation.
03
Faculty members submitting grant applications that involve human subjects.
04
Any staff involved in research protocols requiring ethical review and oversight.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send cumc irb aaaq3153 to be eSigned by others?
To distribute your cumc irb aaaq3153, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
Can I edit cumc irb aaaq3153 on an Android device?
With the pdfFiller mobile app for Android, you may make modifications to PDF files such as cumc irb aaaq3153. Documents may be edited, signed, and sent directly from your mobile device. Install the app and you'll be able to manage your documents from anywhere.
How do I fill out cumc irb aaaq3153 on an Android device?
Complete cumc irb aaaq3153 and other documents on your Android device with the pdfFiller app. The software allows you to modify information, eSign, annotate, and share files. You may view your papers from anywhere with an internet connection.
What is cumc irb aaaq3153?
cumc irb aaaq3153 refers to a specific form or document related to the Institutional Review Board (IRB) at Columbia University Medical Center (CUMC), typically used for research protocol submissions or compliance.
Who is required to file cumc irb aaaq3153?
Researchers or investigators conducting studies involving human subjects at CUMC are required to file cumc irb aaaq3153.
How to fill out cumc irb aaaq3153?
To fill out cumc irb aaaq3153, researchers must provide detailed information about the study design, objectives, participant recruitment, informed consent processes, and any potential risks involved, following the guidelines provided by the CUMC IRB.
What is the purpose of cumc irb aaaq3153?
The purpose of cumc irb aaaq3153 is to ensure that research projects adhere to ethical standards and protect the rights and welfare of human subjects involved in research.
What information must be reported on cumc irb aaaq3153?
The information that must be reported on cumc irb aaaq3153 includes study title, principal investigator details, objectives, methods, risks, benefits, informed consent procedures, and data management plans.
Fill out your cumc irb aaaq3153 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Cumc Irb aaaq3153 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.