Get the free Fda 2301
Show details
Este formulario es utilizado para la presentación de informes periódicos y material promocional relacionado con nuevos medicamentos para animales, requerido por la regulación 21 CFR 514.80. Los
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign fda 2301

Edit your fda 2301 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda 2301 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing fda 2301 online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit fda 2301. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out fda 2301

How to fill out fda 2301
01
Obtain a copy of the FDA Form 2301 from the FDA website or related sources.
02
Begin filling out the form with your contact information, including name, address, phone number, and email.
03
Provide details about the product, including its name, description, and intended use.
04
Indicate the type of application you are submitting (e.g., original submission, amendment, etc.).
05
Complete sections regarding any previous submissions or related applications.
06
Review your entries for accuracy and completeness before submitting.
07
Sign and date the form to authenticate the submission.
Who needs fda 2301?
01
Manufacturers seeking to submit a product for FDA review.
02
Importers looking to register products with the FDA.
03
Distributors involved in the production or sale of regulated products.
04
Any business wanting to ensure compliance with FDA regulations for their products.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my fda 2301 in Gmail?
In your inbox, you may use pdfFiller's add-on for Gmail to generate, modify, fill out, and eSign your fda 2301 and any other papers you receive, all without leaving the program. Install pdfFiller for Gmail from the Google Workspace Marketplace by visiting this link. Take away the need for time-consuming procedures and handle your papers and eSignatures with ease.
Where do I find fda 2301?
It's simple using pdfFiller, an online document management tool. Use our huge online form collection (over 25M fillable forms) to quickly discover the fda 2301. Open it immediately and start altering it with sophisticated capabilities.
How can I fill out fda 2301 on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your fda 2301 from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
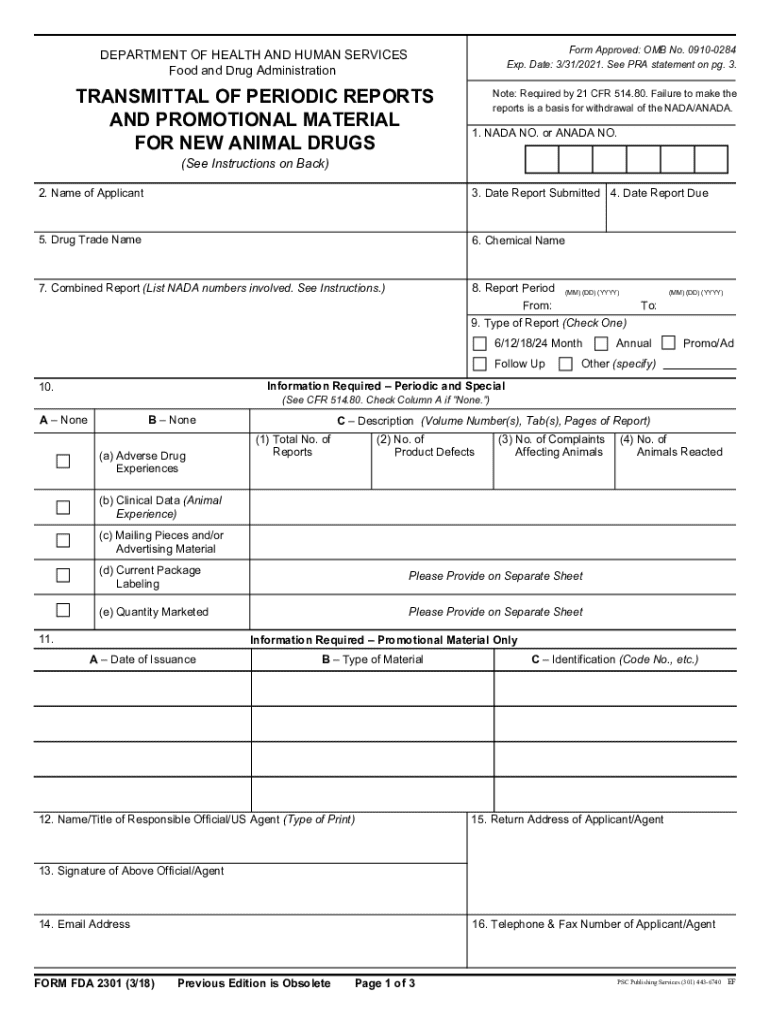
What is fda 2301?
FDA 2301 is a form used for reporting information related to the establishment, maintenance, and operation of a food facility under the jurisdiction of the Food and Drug Administration (FDA).
Who is required to file fda 2301?
Food facilities that are engaged in the manufacturing, processing, packing, or holding of food for consumption must file FDA 2301.
How to fill out fda 2301?
To fill out FDA 2301, individuals must provide information such as the facility name, address, owner details, types of food handled, and operational activities. It is recommended to follow the guidelines provided by the FDA.
What is the purpose of fda 2301?
The purpose of FDA 2301 is to ensure that the FDA has current and accurate information about food facilities to monitor and regulate food safety and compliance.
What information must be reported on fda 2301?
The information that must be reported on FDA 2301 includes facility name, location, type of business, owner information, food products handled, and any relevant contact information.
Fill out your fda 2301 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Fda 2301 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.