Get the free Redox Reactions
Show details
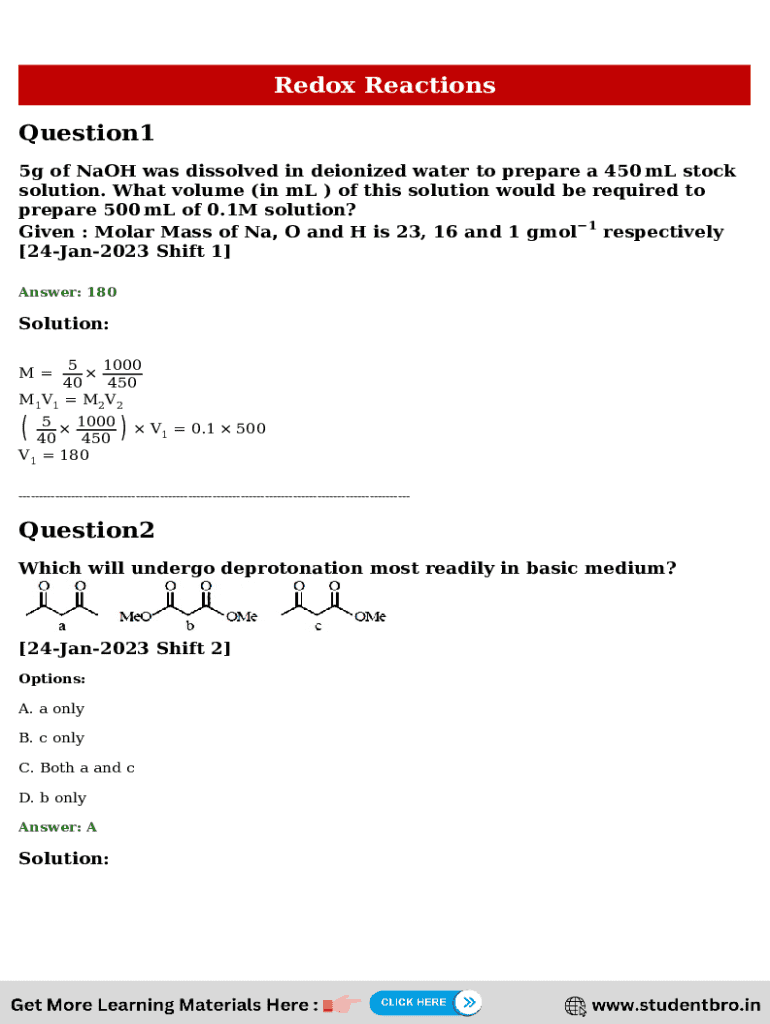
Redox Reactions Question1 5g of NaOH was dissolved in deionized water to prepare a 450 mL stock solution. What volume (in mL ) of this solution would be required to prepare 500 mL of 0.1M solution?
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign redox reactions

Edit your redox reactions form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your redox reactions form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit redox reactions online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to your account. Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit redox reactions. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out redox reactions

How to fill out redox reactions
01
Identify the oxidation and reduction half-reactions.
02
Assign oxidation states to all elements in the reaction.
03
Determine which species is being oxidized and which is being reduced.
04
Balance atoms in each half-reaction, starting with elements other than hydrogen and oxygen.
05
Balance oxygen atoms by adding water (H2O) to the side that needs oxygen.
06
Balance hydrogen atoms by adding hydrogen ions (H+) to the side that needs hydrogen.
07
Balance the charge by adding electrons (e-) to the side that is more positive.
08
Multiply the half-reactions by integers, if necessary, to equalize the number of electrons.
09
Add the half-reactions together and simplify, canceling out common species.
10
Verify that all atoms and charges are balanced in the final redox reaction.
Who needs redox reactions?
01
Chemists for conducting experiments and reactions.
02
Environmental scientists for studying processes like corrosion.
03
Biochemists for understanding metabolic pathways.
04
Industrial manufacturers for processes such as electroplating and battery production.
05
Students and educators in chemistry courses.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find redox reactions?
It's simple using pdfFiller, an online document management tool. Use our huge online form collection (over 25M fillable forms) to quickly discover the redox reactions. Open it immediately and start altering it with sophisticated capabilities.
Can I create an electronic signature for the redox reactions in Chrome?
Yes. By adding the solution to your Chrome browser, you may use pdfFiller to eSign documents while also enjoying all of the PDF editor's capabilities in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a photo of your handwritten signature using the extension. Whatever option you select, you'll be able to eSign your redox reactions in seconds.
How do I edit redox reactions straight from my smartphone?
You can do so easily with pdfFiller’s applications for iOS and Android devices, which can be found at the Apple Store and Google Play Store, respectively. Alternatively, you can get the app on our web page: https://edit-pdf-ios-android.pdffiller.com/. Install the application, log in, and start editing redox reactions right away.
What is redox reactions?
Redox reactions, short for reduction-oxidation reactions, are chemical reactions in which the oxidation states of one or more substances change due to the transfer of electrons. In these reactions, one substance is reduced (gains electrons) while another is oxidized (loses electrons).
Who is required to file redox reactions?
Individuals or organizations engaged in chemical manufacturing, processing, or handling substances that undergo redox reactions may be required to file reports related to these reactions, particularly if they involve regulatory compliance concerning environmental impact or safety.
How to fill out redox reactions?
To fill out redox reactions, identify the reactants and products, determine their oxidation states, balance the equation to ensure mass and charge conservation, and clearly indicate the species that are oxidized and reduced in the reaction.
What is the purpose of redox reactions?
The purpose of redox reactions includes energy production, synthesizing complex molecules, and facilitating various biological and industrial processes. They are fundamental in batteries, corrosion, photosynthesis, and cellular respiration.
What information must be reported on redox reactions?
The information that must be reported includes the reactants and products involved, their oxidation states, the balanced chemical equation, the types of processes (oxidation and reduction), and any pertinent safety or regulatory information related to the substances.
Fill out your redox reactions online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Redox Reactions is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















