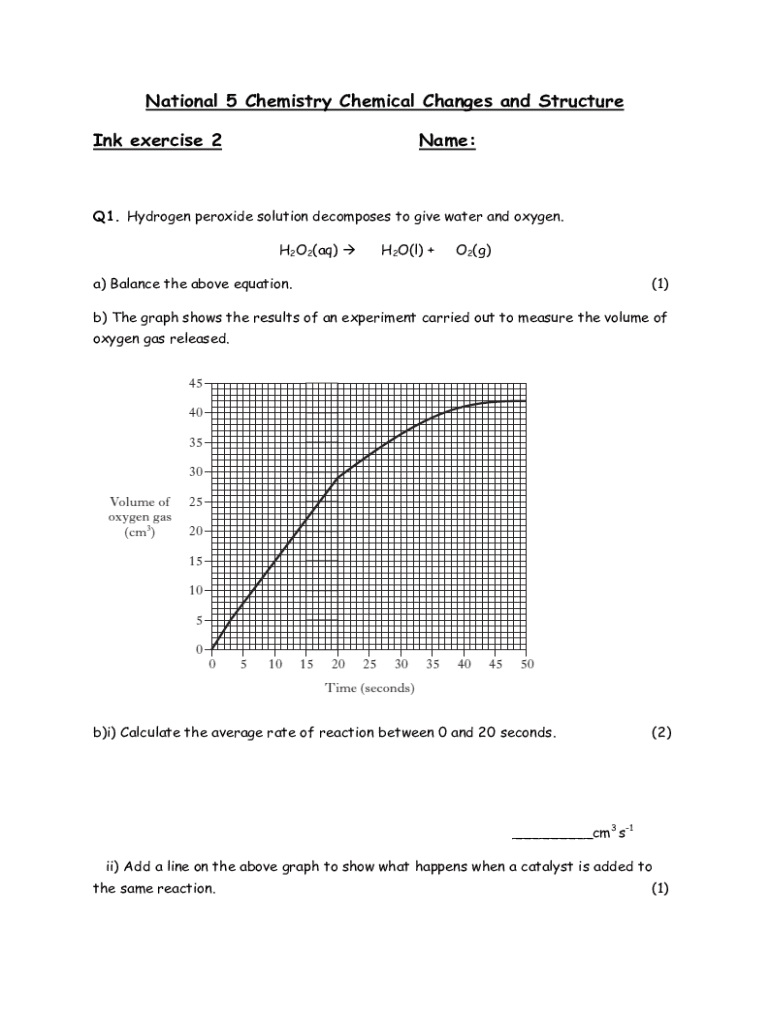
Get the free National 5 Chemistry Chemical Changes and Structure
Get, Create, Make and Sign national 5 chemistry chemical



How to edit national 5 chemistry chemical online
Uncompromising security for your PDF editing and eSignature needs
How to fill out national 5 chemistry chemical

How to fill out national 5 chemistry chemical
Who needs national 5 chemistry chemical?
National 5 Chemistry Chemical Form: A Comprehensive Guide
Understanding chemical formulas
Chemical formulas are vital components in the study of chemistry, serving as shorthand representations of the substances involved in chemical reactions. At their core, these formulas indicate the elements that make up a compound as well as their proportions. In chemistry, we distinguish among elements, compounds, and mixtures. Elements are pure substances that cannot be broken down further, while compounds consist of two or more elements chemically combined. Mixtures, in contrast, contain two or more substances that are not chemically bonded, preserving their individual properties.
The importance of chemical notation cannot be overstated: it enables scientists to communicate complex information succinctly and accurately. These notations allow chemists to understand and predict the behavior of substances, facilitating research and practical applications across various fields.
Key concepts in National 5 chemistry
A foundational understanding of atomic structure is crucial for grasping chemical formulas. Atoms comprise three primary particles: protons, neutrons, and electrons. Protons carry a positive charge and reside in the atomic nucleus alongside neutrons, which have no charge. Electrons, possessing a negative charge, orbit the nucleus, and their arrangement plays a pivotal role in determining an atom's chemical behavior and bonding capabilities. This atomic structure directly influences how elements interact and form compounds, making it essential for successful chemistry comprehension.
The periodic table serves as a vital reference tool in chemistry, organizing elements based on their atomic number, and categorizing them into groups and periods. Each group often shares similar chemical properties due to the arrangement of their outermost electrons, or valence electrons, which are instrumental in chemical bonding. Understanding how to identify elements through their symbols and determining their valency (the capacity of an atom to combine with others) provides the groundwork for formulating chemical equations and oaths.
Writing chemical formulas
Writing chemical formulas involves a systematic approach, starting with identifying the ions involved in a compound and their respective charges. For ionic compounds, a metal and non-metal typically combine, with the metal forming a positively charged cation and the non-metal forming a negatively charged anion. To create a balanced formula, one must ensure that the total positive charge equals the total negative charge, thus achieving electrical neutrality.
A step-by-step approach to writing chemical formulas includes determining the ions from the periodic table, predicting the charge based on the position in the table, and ensuring that the ratio of ions maintains the charge balance. For example, in sodium chloride (NaCl), sodium (Na) has a charge of +1, and chloride (Cl) has a charge of -1, resulting in a 1:1 ratio of ions.
Practical applications of chemical formulas
The role of chemical formulas extends beyond academic study into practical applications, primarily in representing chemical reactions. In these reactions, substances, known as reactants, undergo transformation to yield products. Writing and balancing chemical equations is essential for predicting the outcomes of these reactions and quantifying reactants and products. For instance, in a combustion reaction, hydrocarbons react with oxygen to produce carbon dioxide and water, illustrating the conservation of mass where the total count of each type of atom remains unchanged before and after the reaction.
Beyond the classroom, chemical formulas impact everyday life. Common processes such as respiration involve the transformation of oxygen and glucose into energy, while combustion in vehicles converts fuel into energy, emitting carbon dioxide and water in the process. Moreover, industries leverage chemical formulas extensively in manufacturing, pharmaceuticals, and food production, ensuring safety and efficiency in mass production.
Tools for managing chemical formulae
In today's educational landscape, technology plays a pivotal role in managing chemical documentation. Leveraging platforms like pdfFiller allows users to create, edit, and e-sign chemistry-related documents effortlessly from any device. This accessibility is crucial for students and educators who often collaborate on projects, share resources, and maintain accurate records of experiments and formulas. The platform simplifies document management, ensuring that learning materials are readily available for study and teaching purposes.
Moreover, interactive tools enhance understanding and visualization of chemical concepts. Digital resources, including apps and online databases, provide dynamic representations of molecular structures and periodic trends, allowing for a more profound learning experience. Utilizing interactive tables and molecular models can transform abstract concepts into tangible knowledge, fostering deeper comprehension and engagement with the subject matter.
Enhancing your learning experience
Mastering chemical formulas is integral to succeeding in chemistry. Employing effective study techniques can greatly enhance learning outcomes. Ways to improve your retention of chemical symbols and formulas include mnemonic devices, flashcards, and practice problems. Regularly timing yourself while memorizing tables of elements and their charges can reinforce your memory, while solving practice problems encourages application of knowledge. Recognizing patterns within the periodic table also aids in memorization, highlighting how similar elements behave in comparable ways.
Collaboration plays a significant role in enhancing your learning experience. Engaging with peers and educators fosters discussion and clarification of complex ideas, leading to a more robust understanding of chemistry. Utilizing digital platforms like pdfFiller for sharing worksheets and collaborative projects can streamline the process, allowing for seamless exchanges of ideas and resources.
Common challenges in understanding chemical formulas
Despite the central role of chemical formulas in chemistry, many learners encounter challenges that hinder comprehension. Misconceptions often arise regarding chemical notation, particularly concerning subscript numbers and coefficients. Some students confuse subscripts, which indicate the number of atoms in a molecule, with coefficients, which specify the number of molecules in a chemical equation. This discrepancy can lead to errors in writing and interpreting formulas. Therefore, clarifying the distinction between these elements is crucial for mastering chemical notation.
To overcome these learning obstacles, utilizing available resources can make a significant difference. Whether through tutoring, online forums, or educational platforms like pdfFiller for document sharing, access to help can facilitate a better grasp of chemical formulas. Encouraging consistent practice and seeking feedback helps solidify understanding and boost confidence in using chemical notation effectively.
Assessing your knowledge of chemical formulas
Assessing your understanding of chemical formulas can guide your study efforts and help identify areas for improvement. Creating self-assessment worksheets utilizing platforms like pdfFiller allows you to generate practice problems tailored to your learning needs. These worksheets can encourage active engagement with the material and facilitate revision. Additionally, exploring interactive quizzes and digital resources can enhance your grasp of chemical concepts, providing immediate feedback on your answers.
Regular assessment not only promotes mastery of chemical formulas but can also foster confidence as you advance in your chemistry studies. Pursuing opportunities for self-testing encourages independent learning and critical thinking, crucial components for success in academics and future career paths that rely on a strong chemistry foundation.
Moving forward with chemistry
Building a solid foundation in chemical formulas is essential for future success in chemistry and related fields. As students progress in their studies, a deep understanding of chemical concepts becomes prominent in more complex topics, such as stoichiometry, reaction kinetics, and thermodynamics. Mastery of chemical notation will not only aid in academic pursuits but also open doors to various career paths, including pharmaceuticals, environmental science, and material engineering.
Students and professionals alike can benefit from comprehensive resources that facilitate continuous learning and adaptation. Embracing tools like pdfFiller to manage documents from anywhere enhances accessibility and convenience, enabling users to focus on what truly matters: understanding and applying chemistry. With a strong grasp of chemical formulas as your cornerstone, you can confidently navigate the fascinating world of chemistry and beyond.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit national 5 chemistry chemical from Google Drive?
Can I create an electronic signature for the national 5 chemistry chemical in Chrome?
How do I edit national 5 chemistry chemical straight from my smartphone?
What is national 5 chemistry chemical?
Who is required to file national 5 chemistry chemical?
How to fill out national 5 chemistry chemical?
What is the purpose of national 5 chemistry chemical?
What information must be reported on national 5 chemistry chemical?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















