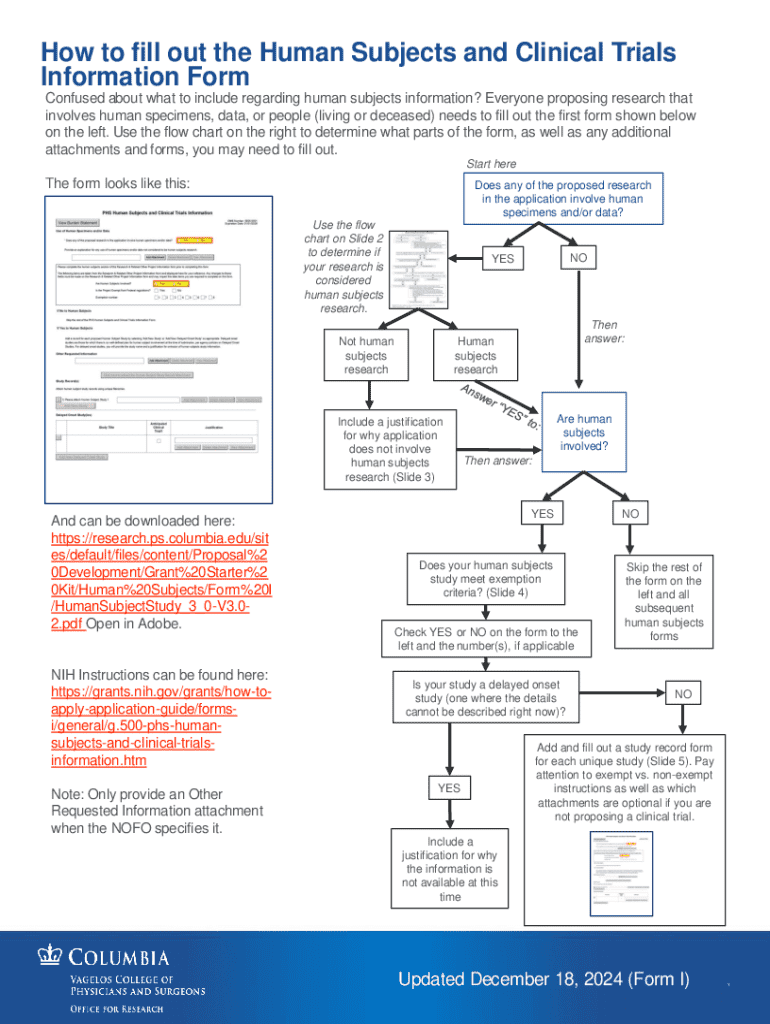
Get the free Human Subjects and Clinical Trials Information Form
Get, Create, Make and Sign human subjects and clinical



Editing human subjects and clinical online
Uncompromising security for your PDF editing and eSignature needs
How to fill out human subjects and clinical

How to fill out human subjects and clinical
Who needs human subjects and clinical?
Human subjects and clinical form: A comprehensive guide for researchers
Overview of human subjects and clinical forms
Human subjects play a crucial role in clinical research as they are the participants whose health outcomes and experiences are investigated to determine the efficacy and safety of new treatments or interventions. This participation is governed by ethical guidelines aimed at protecting the rights and welfare of individuals involved in research. Understanding the clinical form, which captures essential information required for ethical review and compliance, is vital for anyone conducting research involving human subjects.
Key components of clinical forms include participant demographics, study protocol details, risk management strategies, and informed consent processes. These elements ensure that research is conducted transparently and responsibly, supporting both regulatory compliance and participant safety.
Purpose of the human subjects and clinical form
The human subjects and clinical form serves multiple purposes in ensuring that research adheres to ethical standards and regulatory requirements. Primarily, it is a tool for research compliance, ensuring that ethical considerations are addressed and documented. Compliance is essential for obtaining necessary approvals from Institutional Review Boards (IRBs) and other regulatory bodies, which review proposals to safeguard participant welfare.
Moreover, the form enhances the integrity of research by fostering clear communication of the study’s design and objectives. This careful documentation helps protect participant safety by ensuring all aspects of the study, including potential risks, are thoroughly evaluated and communicated to participants.
Accessing the human subjects and clinical form
Researchers can access the human subjects and clinical form through several reliable channels. Government resources such as the National Institutes of Health (NIH) or the Food and Drug Administration (FDA) offer official templates and guidelines for these forms. These agencies provide standardized formats that researchers can adopt to ensure compliance with national standards.
Additionally, many research institutions have their own internal forms tailored to their specific needs. Researchers should consult their institution’s research office or compliance office to obtain the most relevant version of the clinical form. This institutional support often includes guidance on further documentation required based on specific studies.
Key sections of the human subjects and clinical form
The human subjects and clinical form typically contains several key sections that capture critical information needed for ethical review.
Completing the human subjects and clinical form
Completing the human subjects and clinical form requires systematic attention to detail to ensure all necessary information is accurately captured. Each section of the form should be filled out comprehensively. Start by carefully reviewing each section’s requirements and guidelines to avoid common mistakes, such as skipping essential details or misinterpreting instructions.
Utilizing interactive tools can significantly ease the completion process. Platforms such as pdfFiller provide templates that simplify form filling, allowing researchers to edit, sign, and manage documents efficiently. Be sure to keep a checklist of what to include in each section and refer to it frequently as you fill out the form.
Specific considerations based on human subjects involvement
If your study involves human subjects, additional documentation may be required to guarantee ethical compliance. This often includes submitting detailed protocols for informed consent, assessments of risk vs. benefit, and any strategies for participant recruitment. Ethical compliance guidelines dictate that these documents must align with federal regulations, ensuring that participants are fully aware of the nature of the research and any potential risks involved.
Conversely, if your research does not involve human subjects, it’s crucial to clearly indicate this on the form. This usually involves submitting a statement confirming no human subjects will be involved, as well as any alternative documentation required by your institutional review board.
Ensuring compliance and ethics in research
Protecting the rights and welfare of human subjects is the cornerstone of ethical research. Researchers must ensure that participant rights are respected throughout the research process, with proper informed consent practices firmly in place. This means providing participants with clear, comprehensive information about the study and obtaining their voluntary consent to participate.
Another critical aspect of compliance is ensuring data privacy and confidentiality. Researchers must outline their strategies for safeguarding participants’ data, which includes secure storage, limited access, and transparency regarding how data will be used post-study. These practices do not only fulfill regulatory requirements but also help foster trust between researchers and participants.
Conducting a review of your completed form
Once the human subjects and clinical form is completed, performing a thorough review is essential. Establishing a self-assessment checklist can help ensure that all information is accurate and meets institutional and regulatory requirements. Key points to check include verifying that all sections are filled out, confirming the accuracy of participant information, and ensuring that signatures and approvals are obtained as necessary.
Reviewing the form with a peer or mentor can add an additional layer of scrutiny, helping identify any potential oversights while also providing constructive feedback for improvement.
Submission process and follow-up
Submission of the human subjects and clinical form can be conducted electronically or through physical mail, depending on the institutional or regulatory requirements. Familiarize yourself with the specific submission guidelines of your respective IRB or research committee to ensure compliance. Electronic submissions often provide immediate confirmation of receipt, making tracking easier than traditional mail.
Once submitted, it’s crucial to be proactive in tracking your submission status. Establish a timeline for follow-up communications, particularly if you do not receive updates within the expected review period. Understanding what comes next, including potential review requests for additional information or clarifications, will ensure smooth progress in your research endeavor.
Troubleshooting common issues
Researchers often encounter various challenges while filling out the human subjects and clinical form. Common issues include misunderstanding section requirements, providing insufficient details, or failing to adhere to submission protocols. Addressing these problems typically involves consulting available FAQs or institutional guidance to resolve information gaps and ensure correct compliance.
For instance, if a particular section remains unclear, reaching out to your institution's IRB or compliance office can clarify the necessary requirements, thereby enhancing the overall quality of your submission.
Related notices and regulatory considerations
Staying updated on current regulations affecting clinical research is vital for researchers. Regulatory bodies frequently amend guidelines that govern the protection of human subjects and clinical trial conduct. Regularly reviewing the guidelines provided by agencies such as the NIH and FDA will ensure you are aware of any changes or updates relevant to your research.
In addition, institutions often have specific policies that may include internal processes differing from national standards. Researchers should verify these internal regulations to align their clinical forms with institutional requirements.
Tools and resources for managing clinical documents
As you navigate the complexities of clinical documentation, leveraging advanced tools can streamline the process. Platforms like pdfFiller empower users to create, edit, and manage documents from a single, cloud-based solution. These tools enable easier collaborations, eSigning capabilities, and automated form-filling options, drastically reducing the time spent on paperwork.
As researchers increasingly transition to digital formats, utilizing cloud-based solutions not only enhances document management efficiency but also improves accessibility, allowing teams to work on submissions from anywhere. Thus, investing in such tools can facilitate a smoother review and submission process.
Community and support
For further assistance, connecting with communities of researchers and professionals can provide invaluable support. Online forums, social media groups, and dedicated platforms often host discussions that can help answer pressing questions regarding the human subjects and clinical form. Engaging with these communities allows you to share experiences, gain insights, and stay informed about best practices in research compliance.
Additionally, many universities and research institutions host workshops or seminars focused on the important aspects of ethical research and data management, presenting opportunities for networking and knowledge sharing.
Final reminders
Diligence in the submission of the human subjects and clinical form cannot be overstated. Every detail counts, and responsibility toward ethical research should be a top priority. Regularly updating one’s understanding of regulatory changes will ensure that research protocols remain compliant and participant welfare remains protected.
As you embark on your research journey, make sure to stay informed, connected, and proactive, ensuring that all necessary documentation is well-prepared and submitted in a timely manner to avoid hurdles down the line.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an eSignature for the human subjects and clinical in Gmail?
How do I edit human subjects and clinical on an iOS device?
How can I fill out human subjects and clinical on an iOS device?
What is human subjects and clinical?
Who is required to file human subjects and clinical?
How to fill out human subjects and clinical?
What is the purpose of human subjects and clinical?
What information must be reported on human subjects and clinical?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















