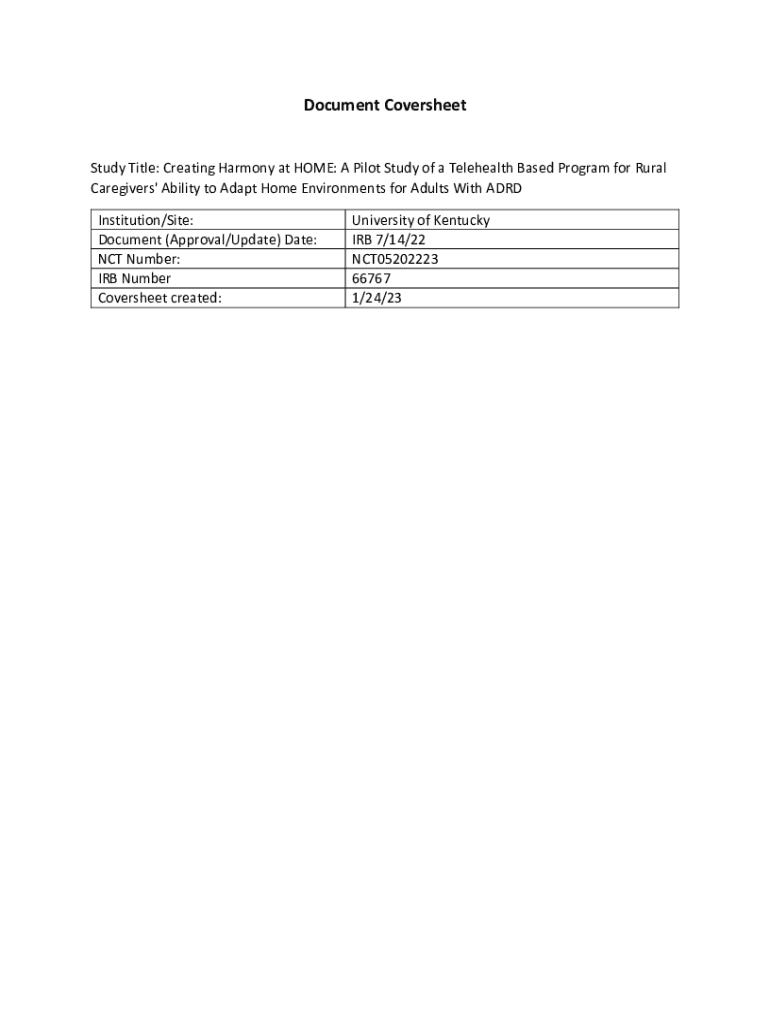
Get the free Consent to Participate in a Research Study
Get, Create, Make and Sign consent to participate in



How to edit consent to participate in online
Uncompromising security for your PDF editing and eSignature needs
How to fill out consent to participate in

How to fill out consent to participate in
Who needs consent to participate in?
Understanding consent to participate in forms: A comprehensive guide
Understanding the importance of consent to participate
A consent to participate form is a crucial document that outlines the rights and responsibilities of participants involved in research or similar activities. It ensures that individuals understand what their participation entails, including any potential risks and benefits. By signing this form, participants grant their permission for researchers to use their data or responses, which is fundamental in maintaining ethical standards in research and documentation.
The importance of such forms cannot be overstated, especially in fields like healthcare, psychology, and social sciences where personal data is collected. Not only does it serve as a legal safeguard for researchers against potential liability, but it also fosters trust and transparency between researchers and participants, thus promoting ethical research practices.
Legal and ethical implications
Obtaining consent is not merely a formality; it is backed by legal requirements that vary by jurisdiction. For instance, in the United States, the Department of Health and Human Services outlines that consent forms must be clear and comprehensive. Ethical considerations also come into play, particularly in safeguarding vulnerable populations, ensuring individuals are not coerced into participation.
Informing participants about how their data will be used is crucial. This transparency helps in making them feel secure about their decisions. Without appropriate consent processes, researchers risk violating ethical guidelines, which can lead to severe consequences for their studies.
Key elements of a consent to participate form
A well-structured consent to participate form includes several required components to ensure clarity and comprehension. One of the most vital elements is an outline of participants' rights. Participants need to know they can withdraw their consent at any time without penalization. Additionally, the purpose of the study or activity must be explicitly stated to give participants a clear understanding of what is expected of them.
Further, a description of any potential risks and benefits must be included. This helps participants weigh their options carefully before consenting. The language used should be plain and understandable, avoiding technical jargon that could confuse someone not versed in the field.
Clear and understandable language
Using clear and simple language is essential to ensure participants fully comprehend what they are signing. Avoiding technical jargon and using everyday language can significantly improve the readability of the consent form. For example, instead of saying 'anonymization of participant data,' one could state 'your personal information will not be shared with anyone.' This approach ensures inclusivity and reduces miscommunication.
Steps to create a consent to participate form
Creating an effective consent to participate form is a systematic process that involves several steps. The first step is to identify the audience. Knowing your target demographic is essential, as it allows you to tailor the content accordingly. For example, consent forms aimed at minors should consider parental consent protocols.
Step two involves drafting the form itself. Include all necessary sections – a clear introduction, participant rights, study purpose, risks, benefits, and a signature line. Ensuring the layout is clear and logically structured will help participants navigate the document effectively.
Reviewing the content
After drafting the document, have peers review it. This not only improves clarity but ensures compliance with ethical guidelines. Utilize peer feedback to clarify confusing sections or restructure parts that lack coherence. Some suggested review questions might include: Is the purpose of the study clear? Are participant rights communicated effectively?
Lastly, incorporate a method for obtaining signatures. Digital options, including eSigning platforms like pdfFiller, simplify the signature collection process while maintaining legal integrity.
Tools for creating and managing consent forms
Utilizing tools like pdfFiller can streamline the creation and management of consent to participate forms. The platform offers interactive templates specifically designed for creating consent forms, which can save time and ensure necessary components are included. Additionally, collaboration features allow multiple team members to provide input, fostering a sense of ownership over the document.
Editing and customizing these templates is straightforward. With pdfFiller’s robust editor, users can easily adjust the wording to match their brand or personalize the document for different studies. This flexibility reduces redundancy and enhances participant engagement in the process.
Best practices for obtaining consent
Engaging participants in the consent process is crucial. To achieve this, clear communication is key. Make it a priority to verbally explain the document if possible, addressing any questions participants may have. This not only helps to clarify misunderstandings but also builds trust. Use strategies such as informative sessions or one-on-one discussions to ensure that participants feel comfortable with the process.
The retention of consent forms is equally important. Storing completed forms securely is critical for compliance and respect towards participants. Using pdfFiller, one can manage and access consent forms easily, ensuring they are appropriately stored and retrievable when needed.
Addressing common concerns and questions
Participants often have questions or concerns regarding the consent to participate form. Common misunderstandings include the belief that signing the form means they are waiving their rights or that they cannot withdraw once they have signed. Clarifying these points upfront can help alleviate hesitation and encourage participation.
If a participant wishes to revoke their consent, it is vital to have a clear procedure in place. Inform participants of their rights to withdraw, and ensure the process is straightforward. Offer reassurances that revoking consent will not lead to any negative consequences.
Advanced considerations in consent processes
When considering the choice between traditional and digital consent forms, there are unique advantages and challenges for each. Digital consent, including options like eSigning, can streamline the process, allowing for quicker access and submission. However, researchers must ensure that electronic signatures meet legal requirements in their jurisdiction.
Cultural sensitivity is another critical factor in the consent process. Understanding and respecting diverse cultural backgrounds can significantly impact how consent is perceived. Adapting forms to ensure they are culturally appropriate helps foster inclusivity and respect, enhancing the experience for participants.
Encouraging communication and feedback
Establishing a feedback loop with participants can enhance the consent process significantly. Feedback helps identify areas for improvement and allows participants to express any concerns they might have. Create easy ways for participants to communicate, whether through follow-up surveys or direct contact. This open dialogue fosters trust and promotes a more positive experience for everyone involved.
Additionally, utilizing participant feedback can lead to more ethical practices in future studies. By continuously refining the consent process based on participant insights, researchers can ensure that consent to participate forms remain effective and respectful.
Conclusion
A well-crafted consent to participate form is an indispensable tool in research and documentation. From ensuring ethical transparency to protecting legal rights, these forms must be created with thoughtful consideration. By following best practices, utilizing modern tools like pdfFiller, and maintaining clear communication with participants, researchers can facilitate a respectful and effective consent process that upholds the integrity of their work and the rights of their participants.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my consent to participate in in Gmail?
How can I edit consent to participate in from Google Drive?
How do I fill out consent to participate in on an Android device?
What is consent to participate in?
Who is required to file consent to participate in?
How to fill out consent to participate in?
What is the purpose of consent to participate in?
What information must be reported on consent to participate in?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















