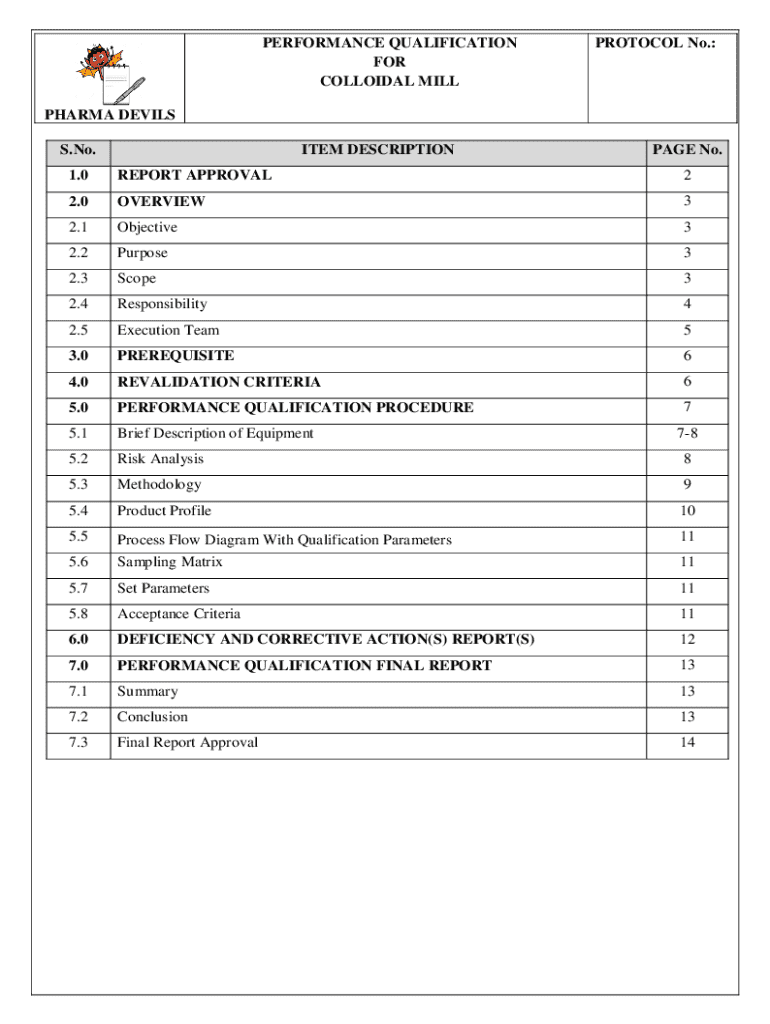
Get the free Performance Qualification for Colloidal Mill
Get, Create, Make and Sign performance qualification for colloidal



Editing performance qualification for colloidal online
Uncompromising security for your PDF editing and eSignature needs
How to fill out performance qualification for colloidal

How to fill out performance qualification for colloidal
Who needs performance qualification for colloidal?
Performance qualification for colloidal form: A comprehensive guide
Understanding performance qualification in colloidal systems
Performance Qualification (PQ) is essential in validating that a product meets its intended use, particularly for colloidal formulations. This process involves the verification of a system's capability to perform effectively and reliably under specified conditions. In colloidal systems, where stability and particle interactions are critical, PQ helps ensure products are not only effective but also safe for consumer usage.
The importance of PQ in colloidal formulations cannot be overstated. It addresses attributes like particle size, distribution, stability, and compatibility. Regulatory bodies such as the FDA and EMA enforce stringent guidelines regarding PQ, necessitating adherence to best practices and documentation to ensure compliance and consumer safety.
Framework for conducting performance qualification
Implementing a robust performance qualification process requires a systematic approach. Start by defining clear objectives and success criteria for your colloidal formulations. This helps in establishing what constitutes acceptable performance, whether it includes stability over time or efficacy in delivering active ingredients.
Collaborating closely with key stakeholders such as R&D, quality assurance, and regulatory affairs is crucial. Each team member plays a specific role in the PQ process, ensuring a comprehensive evaluation of the colloidal system. Regular communication and documentation align efforts towards shared goals.
Testing methodologies for colloidal forms
Performance qualification for colloidal forms typically includes several tests aimed at assessing the product's physical and chemical stability. Key tests involve physical characterization, such as measuring particle size and observing aggregation. Ensuring these parameters meet the defined criteria is fundamental to validating product efficacy.
Developing Standard Operating Procedures (SOPs) for these tests promotes consistency. It’s essential to follow best practices, ensuring reliability and reproducibility in all PQ activities.
Data management and analysis
Comprehensive documentation of performance qualification results is vital. This ensures regulatory compliance and supports future decision-making processes. Utilizing tools like pdfFiller simplifies the management of documents, making it easier to store, retrieve, and share documentation related to PQ.
Adopting statistical methods for interpreting PQ data is essential. Basic techniques such as regression analysis or ANOVA can provide insights into data trends and anomalies. Additionally, employing visualization tools can enhance data comprehension, helping teams identify areas requiring attention.
Challenges and solutions in PQ for colloidal forms
Common hurdles in performance qualification include variability in sample preparation, environmental factors impacting test results, and potential regulatory non-compliance. These challenges can lead to delays in product development and compromise product integrity.
Case studies illustrating successful PQ implementations can provide valuable insights. For instance, a pharmaceutical company that overcame stability issues in its colloidal product through rigorous PQ protocols can serve as a model for others tackling similar challenges.
Regulatory considerations and compliance
Several regulatory bodies govern performance qualification in pharmaceuticals and consumer products. Understanding guidelines from organizations like the FDA or EMA is essential to developing compliant PQ processes. These guidelines often detail specific requirements for documenting stability and efficacy in colloidal systems.
Maintaining an up-to-date knowledge of these regulations is crucial for companies involved in colloidal product development. Proactive efforts to train staff on regulatory expectations can significantly reduce the risk of non-compliance issues.
Collaborating with interactive tools
Collaborative efforts are enhanced through the use of interactive tools like pdfFiller, which enables team members to engage in document creation and editing in real-time. Features such as commenting and markup capabilities allow for efficient feedback and collaboration, reducing the time spent in review cycles.
Using interactive templates can streamline document management, ensuring that all stakeholders have access to the most current versions of PQ documents, facilitating better decision-making and enhancing overall project efficiency.
Finalizing performance qualification reports
A well-structured performance qualification report is fundamental in documenting the PQ process. Key components include detailed descriptions of testing methodologies, results, conclusions, and recommendations for future actions. Proper documentation aids in transparency and accountability.
Establishing a clear review and approval process ensures that all reports are vetted thoroughly before distribution. Archiving PQ documents effectively supports compliance audits and provides a resource for future product evaluations.
Future trends in performance qualification for colloidal systems
The landscape of performance qualification for colloidal systems is evolving with rapid advancements in technology. Innovations such as AI-driven data analysis and predictive modeling are beginning to streamline the PQ process, reducing time and increasing accuracy in results.
As industry standards continue to shift, organizations must stay informed about emerging trends and adapt their PQ methodologies accordingly. Expect shifts in regulatory landscapes aimed at enhancing product safety and efficacy, which will directly impact PQ requirements.
Integrating performance qualification in product development strategies
Aligning performance qualification with overall project management strategies amplifies its impact on product development. Developing long-term strategies that encompass continuous improvement in PQ enables teams to respond efficiently to changes in regulations or consumer expectations.
Regular internal reviews of PQ outcomes and integrating feedback into the development process fosters a culture of quality and innovation, ultimately enhancing product reliability in the marketplace.
Additional considerations and resources
To optimize performance qualification workflows, utilizing tools provided by pdfFiller can significantly enhance efficiency. Resources that offer insights into emerging research can guide teams in refining their processes, while connections with industry experts can provide practical advice for tackling specific challenges during PQ.
Keeping abreast of these additional resources not only empowers teams but also ensures the successful implementation of PQ strategies, ultimately leading to better product development outcomes.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete performance qualification for colloidal online?
Can I sign the performance qualification for colloidal electronically in Chrome?
How do I complete performance qualification for colloidal on an iOS device?
What is performance qualification for colloidal?
Who is required to file performance qualification for colloidal?
How to fill out performance qualification for colloidal?
What is the purpose of performance qualification for colloidal?
What information must be reported on performance qualification for colloidal?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

























