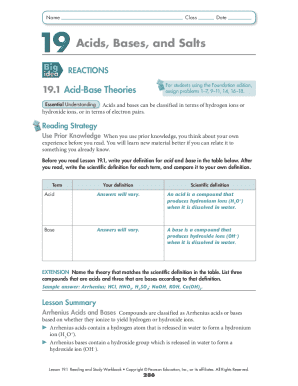
Get the free Study Protocol for Effects of Photobiomodulation on Re-epithelialization of Burn Wound
Get, Create, Make and Sign study protocol for effects



Editing study protocol for effects online
Uncompromising security for your PDF editing and eSignature needs
How to fill out study protocol for effects

How to fill out study protocol for effects
Who needs study protocol for effects?
Study protocol for effects form: A comprehensive guide
Overview of the study protocol for effects form
A study protocol serves as the cornerstone of any research project, defining the objectives, design, methodology, and ethical considerations. Within this framework, the effects form plays a crucial role, documenting the anticipated impacts of the study. The effective management and documentation of these protocols are enhanced significantly through tools like pdfFiller, which allow researchers to edit, share, and manage documents in a streamlined and accessible manner.
Understanding the purpose and use of the effects form
The effects form is a specific component of the study protocol that outlines the anticipated outcomes and their implications. This document is relevant in both clinical and non-clinical studies, serving as a safeguard for research compliance and data integrity. A robust effects form typically includes key components such as study objectives, expected outcomes, and methodological considerations for data analysis.
Step-by-step guide to filling out the effects form
Completing an effects form is an essential task that requires attention to detail and comprehensive information. The process begins with identifying all necessary information your study requires. Setting up a pdfFiller account can streamline the documentation process.
Practical considerations when using the effects form
Documenting effects can present various challenges, including ensuring compliance with regulations and efficiently managing time. Utilizing pdfFiller can mitigate these challenges effectively. To maintain compliance, it's crucial to understand institutional standards and incorporate them into the effects form.
Ethical considerations and informed consent
Ethics in research protocols cannot be overstated. The effects form should include provisions for participant consent, detailing how their data will be handled and the implications of their participation. pdfFiller facilitates the management of consent documentation, ensuring all ethical guidelines are adhered to.
Collaboration with other researchers or institutions
When conducting multi-institution research, sharing the effects form with external parties can improve collaborative outcomes. Best practices involve utilizing pdfFiller’s sharing features to ensure that all researchers have access to the latest version of the document. Effective communication remains crucial for successful collaboration.
Quality assurance in protocol submission
Before submission, ensuring the effects form meets quality standards is essential. Implementing quality control strategies can significantly enhance the reliability of the protocol. pdfFiller provides tools to track revisions and changes, fostering a thorough review process.
Expected outcomes and reporting philosophy
Articulating expected effects within the study protocol is crucial for setting clear research goals. Creating a framework for reporting findings effectively ensures that stakeholders are informed in a timely manner. Using pdfFiller, researchers can present these outcomes with professionalism.
Managing data and statistical analysis
A well-defined framework for data management related to the effects form supports accurate analysis. By integrating pdfFiller into your workflow, you can create data collection forms that facilitate broader research impact while ensuring that collected data is organized for rigorous statistical analysis.
Ongoing monitoring and follow-up procedures
Establishing follow-up protocols is vital for assessing ongoing effects post-study. Utilizing pdfFiller to implement follow-up documentation helps maintain comprehensive tracking of participant responses, allowing for effective longitudinal studies.
Overcoming anticipated challenges
Challenges in effects documentation, such as data inconsistencies, can impede research progress. Adopting resources available through pdfFiller can provide solutions that minimize these obstacles. Seeking support from the pdfFiller community can foster collaborative problem-solving.
Advanced tools for research efficiency
pdfFiller offers additional tools that streamline research processes, including template management for future protocols and forms for data collection. Integrating these resources into existing workflows can significantly enhance efficiency, enabling researchers to focus on their core objectives.
Feedback mechanisms and continuous improvement
Collecting feedback on the effects form process promotes continuous improvement in research practices. By leveraging tools within pdfFiller, researchers can iterate on their protocols based on this feedback, ensuring optimal outcomes in future studies.
Engaging with the research community
Participating in discussions about the effectiveness of the effects form with the research community can heighten awareness and collaboration. pdfFiller provides resources for connecting with peers, sharing insights, and promoting collaborations that lead to shared success in research initiatives.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my study protocol for effects directly from Gmail?
How do I edit study protocol for effects in Chrome?
How do I complete study protocol for effects on an Android device?
What is study protocol for effects?
Who is required to file study protocol for effects?
How to fill out study protocol for effects?
What is the purpose of study protocol for effects?
What information must be reported on study protocol for effects?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.