Get the free Transfusion Reaction Report Form - d2xk4h2me8pjt2 cloudfront
Get, Create, Make and Sign transfusion reaction report form



Editing transfusion reaction report form online
Uncompromising security for your PDF editing and eSignature needs
How to fill out transfusion reaction report form

How to fill out transfusion reaction report form
Who needs transfusion reaction report form?
Transfusion reaction report form: A comprehensive how-to guide
Understanding transfusion reactions
A transfusion reaction is a significant adverse event occurring during or after blood transfusion, manifesting as various symptoms that can impact patient health. These reactions can stem from different causes, including immune responses to transfused blood components or infections. Common causes include mismatched blood types, allergic reactions, and transfusion-associated lung injury. Understanding the various types of transfusion reactions is crucial; they can be categorized into acute reactions, occurring within 24 hours of transfusion, and delayed reactions, which manifest days to weeks later.
Reporting transfusion reactions is essential not just for immediate patient safety but also for refining transfusion practices across the healthcare landscape. By tracking adverse events, healthcare professionals can identify trends, implement corrective measures, and enhance protocols, ultimately improving patient outcomes.
The transfusion reaction report form
The transfusion reaction report form serves a dual purpose: documenting adverse reactions and ensuring compliance with healthcare regulations. This form is pivotal in capturing crucial data that can help assess the safety of blood transfusion practices. Clear documentation allows for systematic evaluations of reactions, facilitating improvements and adherence to regulatory standards.
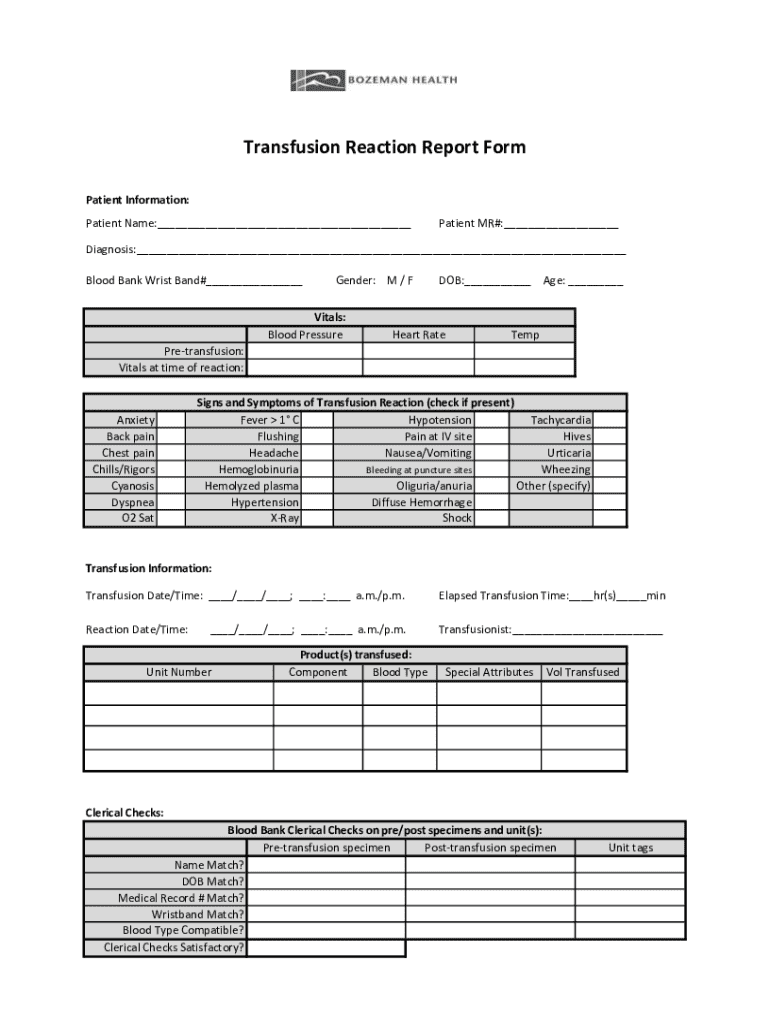
Key components of the transfusion reaction report form include patient information, transfusion details, clinical assessment findings, a thorough description of the reaction, and follow-up actions taken. By capturing these elements comprehensively, healthcare providers can outline the sequence of events leading to the reaction, ensuring a well-rounded understanding of the patient's response.
Step-by-step guide to filling out the report form
Before filling out the report form, it’s imperative to gather all necessary information. Understanding the patient’s transfusion history and current condition will enable accurate documentation. This may involve reviewing medical records, discussing with nursing staff, and assessing vital signs before the transfusion.
1. **Patient information**: Collect accurate demographic details like name, date of birth, and identification numbers. Ensure the information matches hospital records to prevent errors. 2. **Transfusion details**: Document specifics such as the type of blood product administered, the date and time of the transfusion, and infusion rates. 3. **Reaction description**: Clearly outline the symptoms experienced. It's essential to include observable signs, while remaining objective in your description. 4. **Clinical assessment findings**: Record vital signs, any symptoms noted during the reaction, and assessments from the healthcare team before and after the incident. 5. **Follow-up actions**: Specify what actions were taken in response to the reaction, including any medical interventions, notifications to the blood bank, or changes in patient treatment plans.
Best practices for reporting transfusion reactions
Accurate reporting is crucial in maintaining patient safety and enhancing transfusion medicine. When filling out the transfusion reaction report form, use clear and concise language to describe events. This clarity can lessen misunderstandings and ensure that all team members know the situation. Additionally, timely reporting is essential; delays can prevent appropriate responses and trend analysis that can save lives.
Common mistakes include omitting crucial details such as the exact time of the transfusion or failing to document follow-up care. Always double-check entries to avoid these pitfalls, as even small omissions can lead to significant challenges in patient safety and record-keeping.
Interactive tools and resources
Utilizing tools such as pdfFiller can significantly improve the efficiency and accuracy of filling out your transfusion reaction report form. With pdfFiller, you can edit PDFs effortlessly, allowing for clear comprehension of templates. This capability is particularly beneficial when you need to adapt forms to reflect institutional requirements or regulatory updates.
Moreover, pdfFiller's eSigning features ensure that report forms are signed digitally, streamlining the approval process and enhancing the traceability of documentation. Collaboration is also made easier; healthcare teams can share reports in real-time, make comments, and track changes, ensuring that everyone stays informed and aligned.
Contributing to haemovigilance efforts
Haemovigilance is a critical component of blood transfusion safety, aimed at monitoring and preventing adverse reactions associated with blood products. Reporting transfusion reactions contributes to international haemovigilance efforts, which help develop guidelines and standards aimed at minimizing patient risk on a global scale.
Engaging with localized haemovigilance initiatives can also enhance reporting quality. Many regions have established protocols for tracking transfusion-related adverse events, and participating in these efforts can provide healthcare professionals with ongoing education and updated information on best practices, ultimately enhancing patient safety.
Case studies and real-life applications
Analyzing successful reporting examples can provide valuable insights into effective transfusion management. For instance, review cases where clear documentation and swift reporting mitigated severe reactions. Learning from these scenarios can highlight the importance of completing the transfusion reaction report form comprehensively. Understanding how specific actions led to improved patient outcomes or refined protocols can motivate healthcare providers to adhere strictly to the reporting process.
Healthcare professionals often share testimonials that highlight their experiences with the transfusion reaction report form. These personal accounts can underscore the significance of accurate reporting, showcasing real-world impacts on patient care and institutional protocols.
Related topics of interest
For those interested in expanding their understanding of transfusion medicine, advanced topics such as immunohematology and transfusion-related immune modulation are worth exploring. Additionally, investigating the role of data analytics in transfusion safety can provide insights into how patterns and trends can be analyzed to enhance patient outcomes.
As the field of haemovigilance evolves, staying informed about current and future trends is critical. Keeping abreast of developments in transfusion practices will not only benefit individual practitioners but also contribute to the collective goal of improving patient safety and care.
Additional tools and information
Accessing report form templates is made easy with tools like pdfFiller, where users can download customizable forms tailored to their specific organization’s needs. This flexibility allows facilities to maintain consistency in documentation while adapting to changes in guidelines or operational procedures.
In a fast-paced environment, staying updated on policies and procedures regarding transfusion reactions is vital. Continuous education ensures that healthcare providers are aware of the latest best practices and regulatory requirements, thereby fostering a culture of safety and diligence in transfusion medicine.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find transfusion reaction report form?
Can I create an electronic signature for the transfusion reaction report form in Chrome?
How do I fill out the transfusion reaction report form form on my smartphone?
What is transfusion reaction report form?
Who is required to file transfusion reaction report form?
How to fill out transfusion reaction report form?
What is the purpose of transfusion reaction report form?
What information must be reported on transfusion reaction report form?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















