Get the free Center for Drug Evaluation and Safety – Data Request
Get, Create, Make and Sign center for drug evaluation



Editing center for drug evaluation online
Uncompromising security for your PDF editing and eSignature needs
How to fill out center for drug evaluation

How to fill out center for drug evaluation
Who needs center for drug evaluation?
Center for Drug Evaluation Form – How-to Guide
Overview of the Center for Drug Evaluation Form
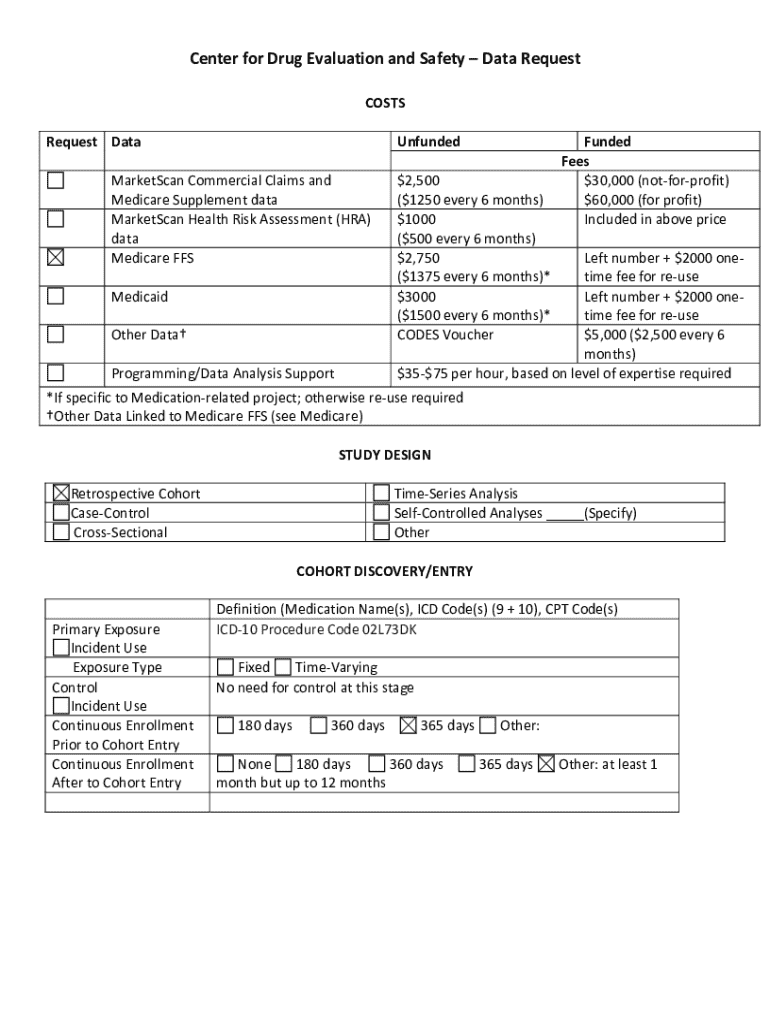
The Center for Drug Evaluation Form is a critical tool utilized in assessing the safety and efficacy of drugs before they reach the market. This form serves as a standardized method for collecting data that regulators and healthcare providers rely on to make informed decisions about drug approvals. Its importance is underscored by the potential impact of new pharmaceuticals on patient health and public safety.
This form is essential for various stakeholders in the healthcare and pharmaceutical industries. Individuals seeking medication, healthcare providers prescribing drugs, and regulatory agencies responsible for drug approvals all interact with this essential documentation. Understanding its usage and significance can streamline the drug evaluation process for all parties involved.
Key features of the Center for Drug Evaluation Form
The Center for Drug Evaluation Form incorporates several key features designed to facilitate the evaluation process. Each form typically includes sections that gather critical information to assess a drug's profile, covering everything from personal details to specific evaluation criteria.
One unique aspect of this form is its interactive tools that enhance the user experience. With customizable sections, users can tailor the form to capture the specific details relevant to their situation, thereby optimizing the evaluation process.
How to access the Center for Drug Evaluation Form
Accessing the Center for Drug Evaluation Form is streamlined through platforms like pdfFiller, which provides a user-friendly interface for locating essential documents. Users can easily navigate to the necessary page on pdfFiller and make use of search functionalities to quickly find the specific form they need.
Moreover, the form is designed to be accessible from various devices including desktops, tablets, and mobile phones. This wide accessibility ensures users can fill out the form anywhere, making it particularly convenient in fast-paced clinical settings.
Detailed instructions for filling out the form
Completing the Center for Drug Evaluation Form requires careful attention to detail. Users should follow a systematic step-by-step approach to ensure that every section is filled out accurately.
Being meticulous with this form is key. Common mistakes include misrepresenting drug history or skipping required fields. Double-checking entries is crucial to avoid delays or complications in the evaluation process.
Editing and customizing the Center for Drug Evaluation Form
pdfFiller offers a suite of editing tools that enable users to customize the Center for Drug Evaluation Form easily. This allows individuals or teams to highlight important sections, annotate as needed, and modify predefined areas to suit their unique requirements.
Once the form is adequately filled out, users have various options for saving and downloading. The document can be exported in multiple formats, ensuring compatibility with different systems or printing requirements.
Signing the Center for Drug Evaluation Form
Once the Center for Drug Evaluation Form has been completed, it will require a signature to validate its contents. pdfFiller facilitates an efficient eSigning process that simplifies the act of officially signing the document without the necessity for physical paperwork.
Users should be aware of the legal considerations associated with eSigning. Typically, eSigned documents carry the same legal weight as traditional signatures, making compliance with eSignature laws critical.
Submitting the Center for Drug Evaluation Form
With the Center for Drug Evaluation Form signed, the next step is submission. Users have options to submit their forms online through pdfFiller or by alternative methods, ensuring flexibility in how the document reaches the necessary authorities.
It is equally important to verify the submission of the form. Users can confirm submissions through receipts generated by pdfFiller or follow-up with the recipient organization to ensure that the form has been received.
Frequently asked questions about the Center for Drug Evaluation Form
Navigating the complexities of the Center for Drug Evaluation Form may raise several questions for users. Addressing these common queries can help users move forward with confidence in their submission process.
For further assistance, users can rely on contact options provided through pdfFiller, ensuring they get the help they need in a timely manner.
Understanding the evaluation process following submission
After submitting the Center for Drug Evaluation Form, it enters a review and validation stage. This phase is crucial for determining the safety and efficacy of the drug in question. Understanding what follows post-submission can prepare users for the possible outcomes of their evaluations.
Approval timelines can vary based on the complexity of the drug and the data provided. Understanding these timelines and expectations can help users navigate subsequent steps after submission.
Importance of keeping records
Maintaining records related to the Center for Drug Evaluation Form is essential, not only for compliance but also for personal reference. Proper document management practices can aid users significantly in their ongoing healthcare or pharmaceutical pursuits.
A well-managed documentation strategy ensures that important information is readily available when needed, preventing unnecessary complications down the line.
Additional services offered by pdfFiller
pdfFiller provides comprehensive document management solutions that go beyond just the Center for Drug Evaluation Form. Users can leverage a variety of services designed to enhance productivity and collaboration within teams.
Utilizing these additional services can significantly streamline workflow and promote efficiency, making pdfFiller an invaluable resource for individuals and teams alike.
User testimonials and success stories
The experiences of users who have effectively utilized the Center for Drug Evaluation Form are invaluable. Testimonials and success stories often highlight how this form facilitated their processes and led to successful drug evaluations.
These accounts serve as motivation for new users and show the practical value of the Center for Drug Evaluation Form in the healthcare landscape.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my center for drug evaluation in Gmail?
Can I create an eSignature for the center for drug evaluation in Gmail?
How do I fill out center for drug evaluation on an Android device?
What is center for drug evaluation?
Who is required to file center for drug evaluation?
How to fill out center for drug evaluation?
What is the purpose of center for drug evaluation?
What information must be reported on center for drug evaluation?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















