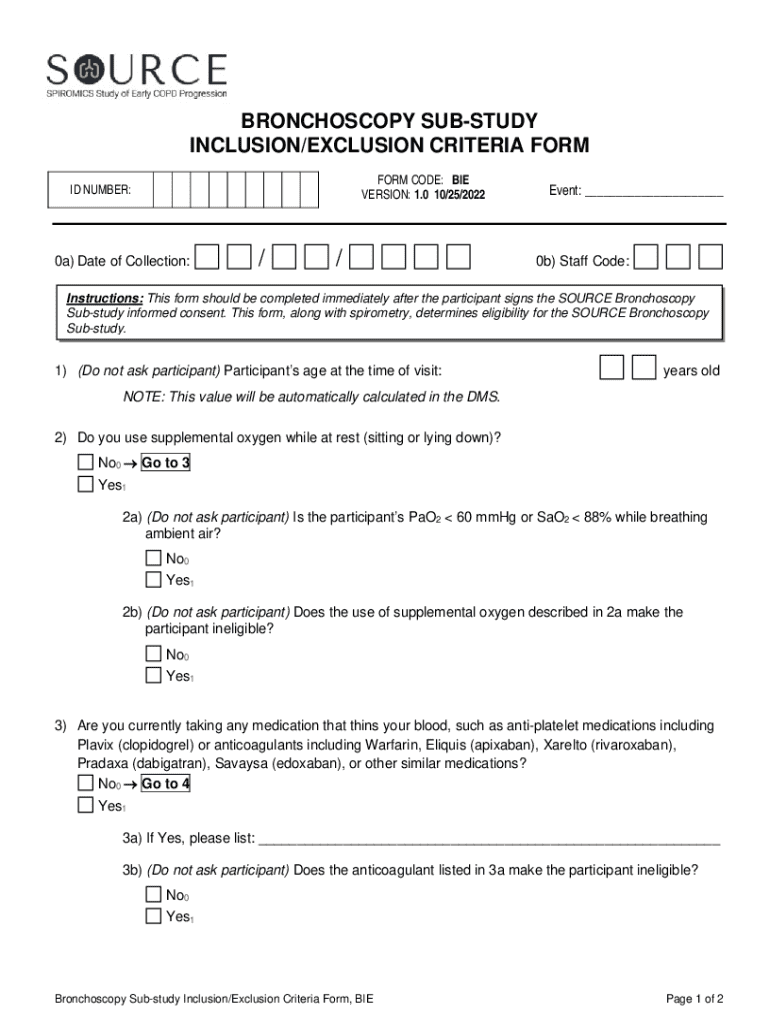
Get the free Bronchoscopy Sub-study Inclusion/exclusion Criteria Form
Get, Create, Make and Sign bronchoscopy sub-study inclusionexclusion criteria



Editing bronchoscopy sub-study inclusionexclusion criteria online
Uncompromising security for your PDF editing and eSignature needs
How to fill out bronchoscopy sub-study inclusionexclusion criteria

How to fill out bronchoscopy sub-study inclusionexclusion criteria
Who needs bronchoscopy sub-study inclusionexclusion criteria?
Comprehensive Guide to the Bronchoscopy Sub-study Inclusion/Exclusion Criteria Form
Overview of the bronchoscopy sub-study inclusion/exclusion criteria form
The bronchoscopy sub-study inclusion/exclusion criteria form plays a crucial role in clinical research, particularly when assessing the eligibility of participants for specific studies. Inclusion and exclusion criteria are fundamental to ensuring that a study yields valid, reliable, and applicable data. These criteria delineate the specific characteristics essential for enrolling participants, thereby streamlining the research process and enhancing safety and effectiveness.
Bronchoscopy sub-studies evaluate various respiratory conditions and the efficacy of interventions or treatments. The inclusion/exclusion criteria serve the dual purpose of identifying optimal candidates for a study while guarding against those whose participation may jeopardize their safety or the integrity of the results.
Key definitions
To understand the bronchoscopy sub-study inclusion/exclusion criteria form, a few key definitions must be made clear. Inclusion criteria specify the requirements participants must meet to be eligible for a study. These criteria can include age ranges, specific health issues, or required medical history. They help researchers target specific populations that may benefit most from the intervention being studied.
Exclusion criteria, on the other hand, are factors that disqualify potential participants. This may include certain existing medical conditions that could complicate the study or put participants at risk. The careful management of these criteria directly impacts the validity of the study's outcomes and the safety of its participants.
Detailed specifications of the inclusion/exclusion criteria
Filling out the bronchoscopy sub-study inclusion/exclusion criteria form requires meticulous attention to a variety of specifications. Age and gender requirements often form the foundation, as certain medications or interventions may only be applicable to specific demographics. For instance, studies may focus on age groups ranging from young adults to elderly patients, ensuring that the results are relevant to the intended population.
Medical history parameters significantly impact eligibility. Conditions such as chronic obstructive pulmonary disease (COPD), asthma, or lung cancer must be carefully considered. Current medications and treatments must also be documented, as they could affect participant safety during the study. Additionally, factors like lifestyle (e.g., smoking status) and occupational exposure can provide critical insights that determine whether an individual qualifies.
Filling out the bronchoscopy sub-study inclusion/exclusion criteria form
Accurately completing the bronchoscopy sub-study inclusion/exclusion criteria form is essential for ensuring valid participation. Begin by gathering all necessary documentation related to personal health and medical history. This will often include medical records, lists of current medications, and information related to lifestyle factors.
Once documentation is in hand, the next step is verifying personal information against the provided criteria. This includes age, gender, and medical conditions. If there's any uncertainty about your medical history or medications, consulting with healthcare providers can provide clarity. The final submissions need to accurately reflect one's eligibility to avoid delays or complications in the enrollment process.
Submission guidelines
Submitting the bronchoscopy sub-study inclusion/exclusion criteria form is a straightforward process, yet it is vital to adhere to specific guidelines. Forms can usually be submitted electronically through clinical trial coordinators or designated research teams. Clear deadlines will be provided, and it’s crucial to recognize these timelines to avoid missing enrollment opportunities.
After submission, the research team will review the forms thoroughly. Participants can expect to receive notifications regarding their eligibility status. If any additional information is needed, coordinators will reach out to discuss next steps. Staying in close communication with the research team can facilitate this process.
Tools for managing your documentation
Utilizing pdfFiller’s cloud-based platform makes managing your bronchoscopy sub-study inclusion/exclusion criteria form simple and efficient. With features for editing PDFs, you can ensure all entries and personal information are accurately captured before submission. Additionally, the collaborative tools allow teams to work together seamlessly, streamlining communication and record management.
eSigning capabilities on pdfFiller enable quick approvals and signature gathering, reducing delays often associated with traditional form management. Users can also track submission status and receive notifications, ensuring that participants stay informed throughout the process.
Frequently asked questions (FAQs)
Addressing common questions about the bronchoscopy sub-study inclusion/exclusion criteria form can provide additional clarity to potential participants. For instance, many wonder what options are available if they do not meet the inclusion criteria. In such cases, researchers may suggest alternative studies or provide insights into why certain criteria exist.
Another frequent query revolves around the possibility of appealing an exclusion decision. In some instances, providing further documentation or clarification might lead to a reconsideration. Criteria updates are also an important topic, as they can change based on evolving research standards, and participants should always be informed. Support is often available for those needing assistance while filling out the form, ensuring a smooth experience.
Contact information for further assistance
For individuals interested in the bronchoscopy sub-study, having easy access to support is essential. Most research teams provide dedicated contact information for participants to address questions or issues that may arise during the process. It's advisable to check with the specific research protocol for detailed contact methods.
Additionally, links to relevant clinical trial information are typically provided, allowing potential participants to become better informed about the studies they are considering. Engaging with accessible support channels can enhance the overall experience and clarify any ambiguities about the inclusion/exclusion criteria form.
Additional forms related to bronchoscopy
In addition to the inclusion/exclusion criteria form, participants may encounter several other important documents related to bronchoscopy research. The bronchoscopy lab ID form is often utilized to link participants to specific studies and facilitate data collection. It is crucial to follow through on all required documentation as each form serves an essential purpose.
Likewise, the bronchoscopy specimen processing worksheet ensures that biological samples are correctly handled and processed according to study protocols. Staying organized with these various documents contributes to the smooth conduct of the study and the safety of its participants.
Related research protocols and citations
Understanding the broader context of bronchoscopy studies involves exploring related research protocols and previous findings. Reviewing pivotal studies in the field can provide insights into how inclusion/exclusion criteria have influenced outcomes over time. Researchers continually refine their approaches based on collective knowledge gained from past experiences.
Important citations and references about previous clinical trials illuminate essential considerations for those involved in bronchoscopy studies. A thorough understanding of related clinical trials can inspire ongoing improvements in research methodologies and highlight areas where further exploration is needed.
Community and support
Participating in bronchoscopy sub-studies is not just a solitary experience. Engaging with a community of participants and researchers can enhance the overall study journey. Accessing an internal login or signing up as a new user facilitates participation in community forums and discussions where experiences can be shared and insights exchanged.
These interactions not only foster a sense of support among participants but can also generate valuable feedback for researchers. Opportunities to engage with others can lead to a deeper understanding of the bronchoscopy processes and the impact of inclusion/exclusion criteria on study results.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete bronchoscopy sub-study inclusionexclusion criteria online?
Can I create an electronic signature for the bronchoscopy sub-study inclusionexclusion criteria in Chrome?
How do I edit bronchoscopy sub-study inclusionexclusion criteria straight from my smartphone?
What is bronchoscopy sub-study inclusion/exclusion criteria?
Who is required to file bronchoscopy sub-study inclusion/exclusion criteria?
How to fill out bronchoscopy sub-study inclusion/exclusion criteria?
What is the purpose of bronchoscopy sub-study inclusion/exclusion criteria?
What information must be reported on bronchoscopy sub-study inclusion/exclusion criteria?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















