Get the free Clinical Research Org & Clinical Trials.doc
Show details
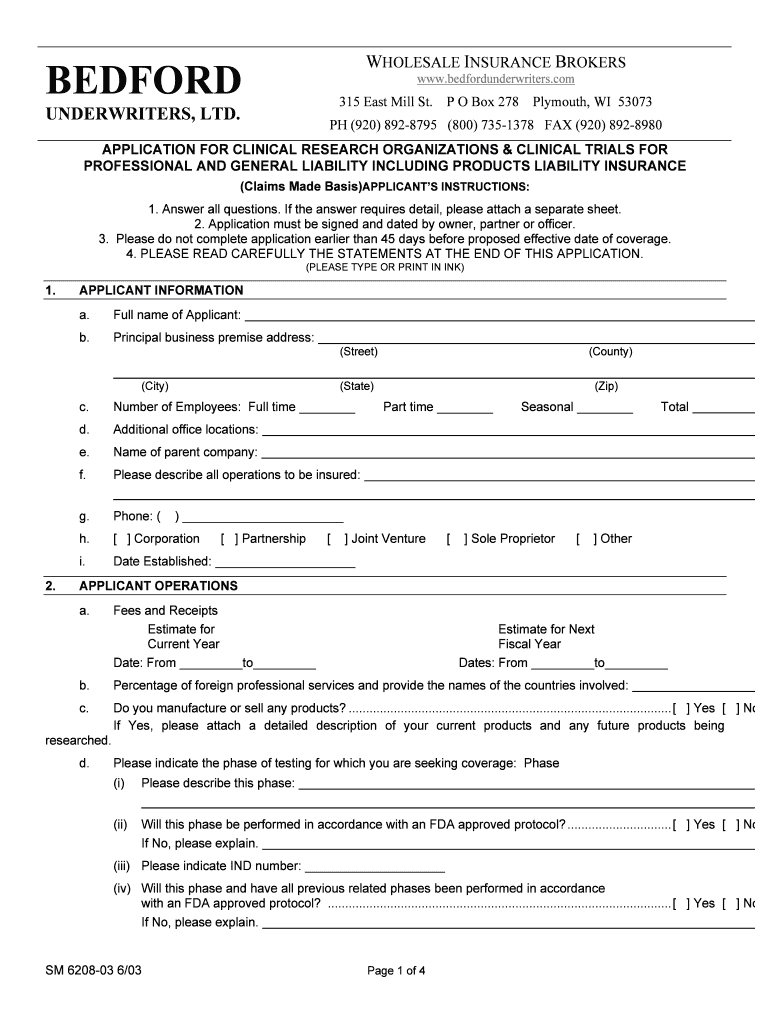
WHOLESALE INSURANCE BROKERS BEDFORD www.bedfordunderwriters.com 315 East Mill St. UNDERWRITERS, LTD. P O Box 278 Plymouth, WI 53073 PH (920) 892-8795 (800) 735-1378 FAX (920) 892-8980 APPLICATION
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical research org amp

Edit your clinical research org amp form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical research org amp form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical research org amp online
To use the professional PDF editor, follow these steps:
1
Log in to your account. Start Free Trial and register a profile if you don't have one yet.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit clinical research org amp. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical research org amp

01
To fill out the clinical research org amp, start by gathering all the necessary information and documents related to your research organization. This may include your organization's name, address, contact information, and any relevant certifications or accreditations.
02
Next, carefully review the instructions provided with the clinical research org amp form. This will help you understand the specific requirements and sections that need to be completed.
03
Begin filling out the form by providing your organization's name, address, and contact information in the designated fields. Make sure to double-check the accuracy of this information to avoid any confusion or potential delays in the process.
04
Depending on the form's requirements, you may need to provide additional details such as your organization's mission statement, research focus areas, and any previous experience or expertise in conducting clinical research.
05
Ensure that you complete all the mandatory sections of the form, providing accurate and up-to-date information. It is important to be thorough and concise in your responses, providing clear and relevant details that showcase your organization's qualifications and capabilities.
06
If there are any specific attachments or supporting documents required, make sure to gather them and submit them along with the completed form. These may include copies of your organization's certifications, licenses, or any relevant research publications.
07
Before submitting the form, carefully review all the information provided to check for any errors or inconsistencies. Mistakes or incomplete information may cause delays or result in the form being rejected.
08
Finally, submit the completed clinical research org amp form through the designated method outlined in the instructions. This can often be done electronically through an online portal or by mailing the physical form to the appropriate regulatory authorities.
Who needs clinical research org amp?
01
Clinical research organizations (CROs) that conduct clinical trials or research studies require the clinical research org amp. This form helps document their organizational information, capabilities, and qualifications in order to comply with regulatory requirements and demonstrate their ability to conduct ethical and reliable research.
02
Research institutions and academic centers that engage in clinical research also need the clinical research org amp. This form helps them showcase their expertise, infrastructure, and adherence to ethical standards, allowing them to collaborate with pharmaceutical companies, healthcare providers, or other stakeholders in the research field.
03
Regulatory bodies and government agencies responsible for overseeing clinical research also rely on the clinical research org amp to evaluate and monitor the organizations involved in clinical trials. This form helps ensure that all research organizations meet the necessary standards and guidelines to protect participant safety and maintain data integrity.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is clinical research org amp?
Clinical research organization (CRO) is a company that offers support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis.
Who is required to file clinical research org amp?
Any company or organization that conducts clinical research studies or trials is required to file a clinical research organization amendment (CRO amp).
How to fill out clinical research org amp?
To fill out a clinical research organization amendment, one must provide detailed information about the study, including protocol changes, sponsor information, and ethical considerations.
What is the purpose of clinical research org amp?
The purpose of a clinical research organization amendment is to ensure that any changes made to a study protocol are properly documented and reviewed to maintain the integrity and validity of the research.
What information must be reported on clinical research org amp?
Information such as changes to study protocols, updated contact information, revised timelines, and any additional funding sources must be reported on a clinical research organization amendment.
How can I send clinical research org amp for eSignature?
Once you are ready to share your clinical research org amp, you can easily send it to others and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail, or notarize it online. You can do all of this without ever leaving your account.
Can I create an eSignature for the clinical research org amp in Gmail?
It's easy to make your eSignature with pdfFiller, and then you can sign your clinical research org amp right from your Gmail inbox with the help of pdfFiller's add-on for Gmail. This is a very important point: You must sign up for an account so that you can save your signatures and signed documents.
How do I fill out clinical research org amp on an Android device?
Use the pdfFiller app for Android to finish your clinical research org amp. The application lets you do all the things you need to do with documents, like add, edit, and remove text, sign, annotate, and more. There is nothing else you need except your smartphone and an internet connection to do this.
Fill out your clinical research org amp online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Research Org Amp is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















