Get the free Chapter 8: Atoms and Periodic Properties Flashcards
Get, Create, Make and Sign chapter 8 atoms and



How to edit chapter 8 atoms and online
Uncompromising security for your PDF editing and eSignature needs
How to fill out chapter 8 atoms and

How to fill out chapter 8 atoms and
Who needs chapter 8 atoms and?
Chapter 8: Atoms and Form
Understanding atoms: The building blocks of matter
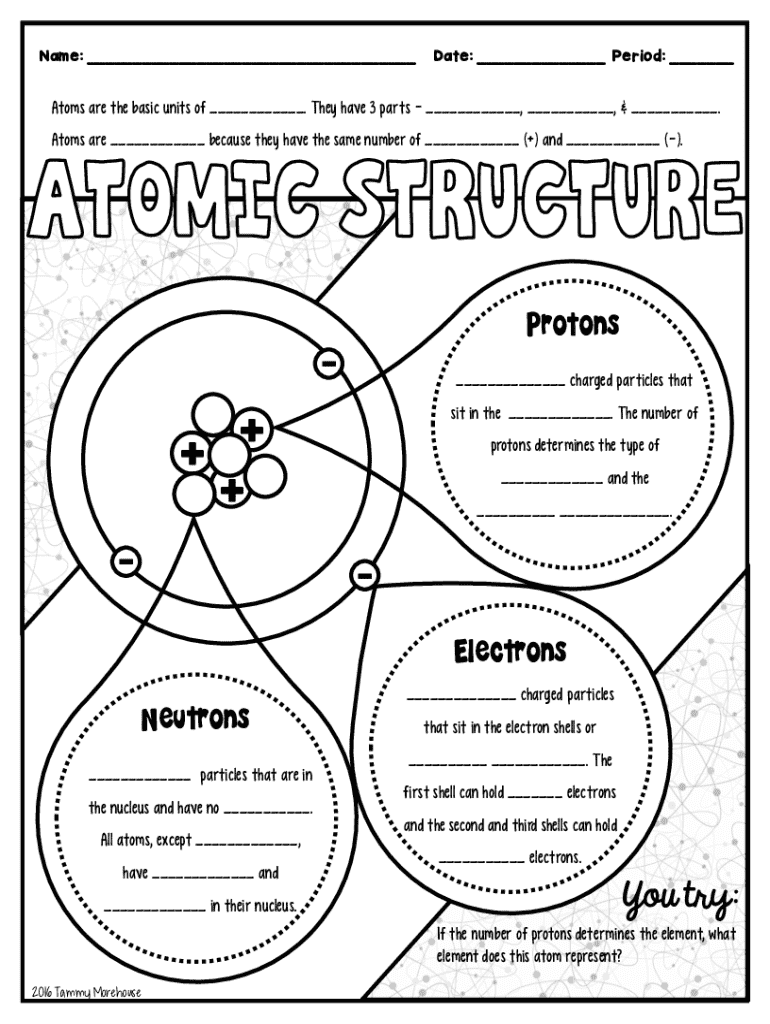
Atoms are the fundamental building blocks of all matter. They are the smallest units of an element that retain the properties of that element. Historically, the concept of the atom dates back to ancient Greece, where philosophers like Democritus proposed that all matter could be divided into indivisible particles. Modern atomic theory, however, recognizes that atoms are composed of even smaller subatomic particles: protons, neutrons, and electrons.
The structure of an atom is elegantly simple yet intricate. It consists of a nucleus at its center, containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons that orbit around the nucleus. Understanding this framework is crucial, as it lays the foundation for exploring chemical reactions and molecular formation.
Molecules: The unions of atoms
Molecules are formed when two or more atoms bond together. This union can involve atoms of the same element, such as in oxygen gas (O2), or different elements, such as in water (H2O). Understanding the distinction between atoms and molecules is vital; while an atom is the basic unit of an element, a molecule represents a combination of these atoms in a specific arrangement.
Molecules can be classified into two main types: simple and complex. Simple molecules, like diatomic oxygen (O2) and nitrogen (N2), consist of just a few atoms. Complex molecules, on the other hand, are made up of a larger number of atoms and can exhibit intricate structures, such as proteins and DNA. Everyday life provides myriad examples of such molecules, emphasizing their ubiquity and importance.
The interaction of atoms and form
Atoms interact through bonding to form molecules. The two primary types of chemical bonding are covalent and ionic. Covalent bonding occurs when atoms share electrons, creating a strong bond indicative of many organic compounds. In contrast, ionic bonding involves the transfer of electrons, resulting in the attraction between positively and negatively charged ions, typical of inorganic compounds like salt.
The arrangement of atoms creates different molecular geometries, which greatly influence the physical and chemical properties of substances. For instance, the bent shape of water contributes to its unique properties, including high surface tension and boiling point, which are vital for sustaining life.
Atomic and molecular mass
Atomic mass is a defined quantity that relates to the mass of an atom. It is typically measured in atomic mass units (amu), where one amu is defined as one twelfth the mass of a carbon-12 atom. Understanding atomic mass is crucial for stoichiometric calculations in chemistry, where ratios of reactants to products are necessary for predicting chemical behaviors.
Molecular mass is determined by summing the atomic masses of all the atoms in a molecule. For example, water's molecular mass can be calculated by adding the masses of two hydrogen atoms and one oxygen atom. This knowledge is essential across various applications, including pharmacology, where dosage calculations are based on molecular weight.
Dalton's atomic theory: Foundations of modern chemistry
John Dalton's atomic theory, proposed in the early 19th century, laid the groundwork for modern chemistry. His key postulates include the belief that elements are composed of atoms which are indivisible, that all atoms of a given element are identical, and that in chemical reactions, atoms are neither created nor destroyed. This foundational perspective underscored the conservation of mass and enabled systematic studies of chemical reactions.
While Dalton’s theory was groundbreaking, advancements in atomic structure, such as the discovery of isotopes and subatomic particles, highlighted its limitations. Newer theories now encompass the dynamic nature of atoms, including their behavior in chemical bonds and reactions, thus expanding our understanding significantly.
The size and scale of atoms
Atomic size is determined by various factors, primarily nuclear charge and electron shielding. The effective nuclear charge influences the attraction between the nucleus and electrons; as protons increase in the nucleus, so does the positive charge, pulling electrons closer and reducing atomic radius. Conversely, electron shielding occurs when inner electrons repel outer electrons, increasing atomic size.
In relativity, the sizes of different atoms vary significantly. For instance, helium has a small atomic radius due to its high nuclear charge relative to its electron count, while francium, the largest atom, has a significant atomic radius due to its lower nuclear charge affecting electron attraction. Understanding these sizes helps in various applications, including predicting bond lengths and molecular shapes.
Common questions about atoms and molecules
As you dive deeper into the world of atoms and form, you might come across several intriguing questions. Can a molecule have only one atom? The answer is affirmative; these are known as monatomic molecules, like noble gases (e.g., helium and neon). Another common query is whether oxygen constitutes a molecule; indeed, as a diatomic molecule (O2), oxygen exists as two atoms bonded together.
How do atoms become molecules? This transformation occurs through chemical bonding, either covalent or ionic, as explored earlier. A key characteristic of atoms noteworthy in this context includes isotopes, which are variants of an element with different numbers of neutrons. Additionally, ions form when atoms gain or lose electrons, resulting in charged particles essential for various chemical processes.
Real-world applications of atomic and molecular understanding
The comprehension of atomic and molecular interactions is integral in everyday life and across various industries. Numerous household products, including cleaners and preservatives, derive from chemical interactions at the atomic level. Moreover, the role of chemistry in medicine cannot be overstated; drug formulation requires detailed knowledge of molecular interactions to ensure efficacy and safety.
In technological advancements, the understanding of atoms and molecules sustains innovation in material science, where new compounds are engineered for specific applications. Looking towards the future, trends in molecular engineering suggest an exciting pathway for developing materials with tailored properties, which can revolutionize sectors ranging from aerospace to electronics.
Visual learning for atoms and molecules
To truly grasp the concepts of atoms and molecules, visual aids can significantly enhance understanding. Interactive tools available online allow users to visualize atomic structures, offering a clearer picture of how atoms bond and interact. These resources can be invaluable for students and enthusiasts alike, allowing for exploration beyond traditional textbooks.
Engaging educational content, such as videos and simulations, provide an interactive approach to learning about molecular chemistry. Such resources reveal dynamic properties of atomic interactions, bringing them to life and making complex ideas more accessible.
Frequently asked questions (FAQs)
Understanding the differences between atoms and molecules is fundamental: atoms are singular, while molecules consist of two or more bonded atoms. Examples of simple molecules include H2 (hydrogen) and O2 (oxygen). What happens to atoms during a chemical reaction? They rearrange through bonding, transforming reactants into products, illustrating the dynamic nature of matter.
Can a molecule be ionic? Yes, ionic compounds are molecules formed from the electrostatic attraction between oppositely charged ions. Finally, in chemistry, the term 'form' refers to the structure and arrangement of molecules, which significantly affect their functions. Understanding these characteristics is crucial for advancing research in chemistry and materials science.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my chapter 8 atoms and in Gmail?
How do I complete chapter 8 atoms and on an iOS device?
Can I edit chapter 8 atoms and on an Android device?
What is chapter 8 atoms and?
Who is required to file chapter 8 atoms and?
How to fill out chapter 8 atoms and?
What is the purpose of chapter 8 atoms and?
What information must be reported on chapter 8 atoms and?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















