Get the free Covid-19 Lab Data Reporting Implementation Specifications - sboh wa
Get, Create, Make and Sign covid-19 lab data reporting



Editing covid-19 lab data reporting online
Uncompromising security for your PDF editing and eSignature needs
How to fill out covid-19 lab data reporting

How to fill out covid-19 lab data reporting
Who needs covid-19 lab data reporting?
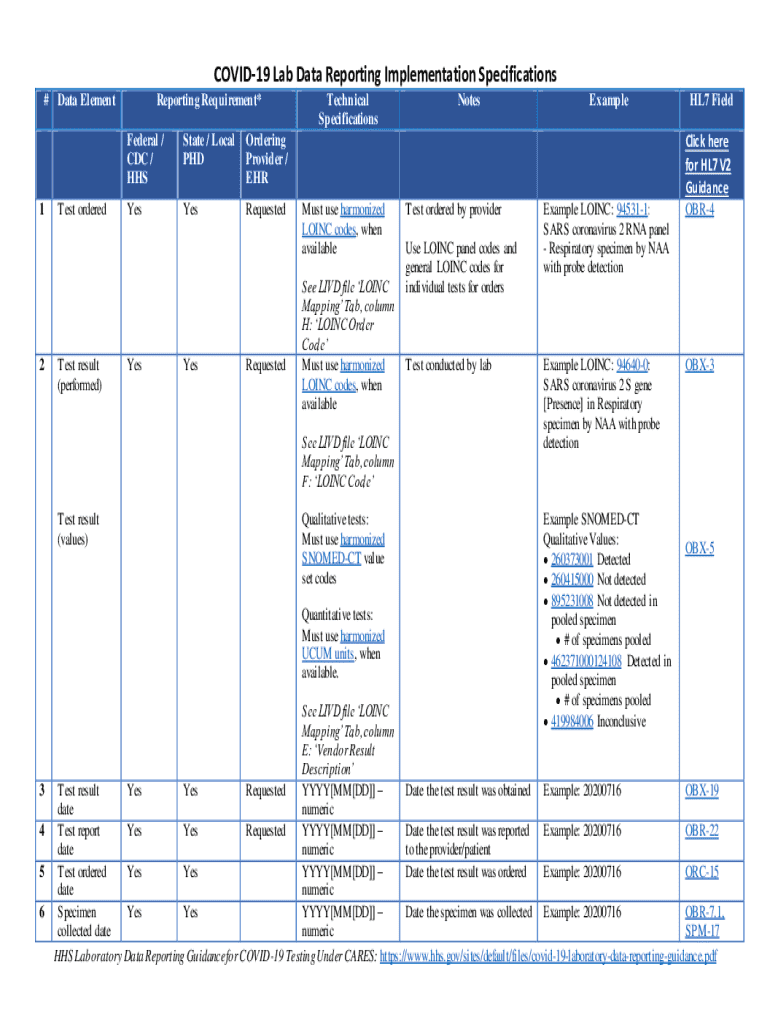
Comprehensive Guide to the COVID-19 Lab Data Reporting Form
Overview of COVID-19 lab data reporting
Laboratory data plays a crucial role in managing the COVID-19 pandemic. Reporting accurate and timely lab results helps public health officials, policymakers, and healthcare providers make data-driven decisions. The COVID-19 Lab Data Reporting Form serves as a standardized tool designed to facilitate the collection and dissemination of lab data related to COVID-19 testing.
Recent changes in reporting guidelines
As the COVID-19 pandemic evolved, so did the guidelines for reporting laboratory data. Recent updates have addressed the need for more comprehensive data collection, including information on variants, vaccination status, and demographic details of tested individuals. These changes are significant as they help capture a fuller picture of community transmission and vaccine effectiveness.
Eligibility criteria for reporters
Not everyone is eligible to report COVID-19 lab data. Generally, certified laboratories and healthcare providers authorized to conduct COVID-19 testing are designated as reporters. This includes hospital laboratories, commercial testing labs, and public health entities.
Step-by-step instructions for filling out the reporting form
Completing the COVID-19 Lab Data Reporting Form can be streamlined by following a structured approach. Here’s a detailed breakdown of the process.
4.1 Accessing the reporting form
To begin, the first step is accessing the COVID-19 Lab Data Reporting Form. You can usually obtain it through the official health department's website or directly from a centralized reporting portal. Make sure you have the most recent version to comply with updated guidelines.
4.2 Required information
The form includes several essential data fields, such as:
4.3 Completing the form
While filling out the form, ensure that all information is accurate and up-to-date. Missing or incorrect information can lead to delays in processing and misinterpretation of data.
4.4 Editing and finalizing the form
Once completed, reviewing and confirming all entries is essential. Double-check that all necessary fields are filled, and consider saving a copy for your records. This ensures no critical data is overlooked before submission.
Utilizing standard terminology for reporting
Using standard terminology when reporting COVID-19 lab data is vital to maintaining consistency and clarity. The CDC and other public health authorities provide guidelines on terminologies to be used, which allows for more reliable data analysis and tracking.
Technical assistance for electronic reporting
For laboratories and healthcare providers transitioning to electronic reporting formats, technical assistance is available. Many health departments have dedicated support lines for queries related to electronic reporting, especially regarding technical issues or specific guidelines.
Resources for reporting COVID-19 data
Numerous resources are available for those tasked with reporting COVID-19 data. Utilizing these resources can greatly enhance the accuracy and efficiency of your reporting efforts.
Frequently asked questions on COVID-19 lab data reporting
It’s common for reporters to have questions regarding the COVID-19 Lab Data Reporting Form. Below are answers to some of the most frequently asked queries.
Reporting COVID-19 test results
Timely reporting of COVID-19 test results is essential for effective public health response. Quick communication allows health officials to initiate necessary measures to contain outbreaks.
Reporting methodologies
Various methodologies are utilized in reporting COVID-19 lab results. Electronic Laboratory Reporting (ELR) has become the standard due to its speed and efficiency. Many laboratories submit results in a format that integrates smoothly with state information systems.
Onboarding process for reporting COVID-19 data
For those new to COVID-19 data reporting, an onboarding process is essential for effective and compliant participation. This ensures that all individuals involved are knowledgeable about the protocols and standards expected.
11.1 Registration
Reporting entities typically need to register with their local health department to gain access to reporting portals and obtain unique identifiers that help track submissions.
11.2 Setting up secure file transfer (sFTP)
Establishing a secure file transfer protocol (sFTP) connection is crucial for protecting sensitive patient information during transmission. Follow guidelines provided by your local health department for setup.
11.3 Testing procedures
Testing the setup before full-scale reporting helps identify and iron out any issues, ensuring a smoother transition to actual reporting efforts.
11.4 Transitioning to production reporting
After successful testing, reporters can transition to full production reporting by adhering to established timelines and processing standards laid out by local health authorities.
Understanding global and national context
The COVID-19 data landscape is continually evolving, with an emphasis on using lab data to understand transmission patterns, vaccine efficacy, and public health response effectiveness. Accurate laboratory reporting is integral to understanding how to tackle future waves.
Data interpretation and correlation
Interpreting reported COVID-19 data involves analyzing trends and correlating lab results with public health policies. This process is essential for understanding the pandemic's trajectory and for formulating future public health strategies.
Contact points for further information
For further inquiries related to the COVID-19 Lab Data Reporting Form, various channels of communication are available to ensure that you receive timely and accurate information.
Subscribe to updates
Staying informed on changes in reporting guidelines is essential for laboratories and healthcare providers. Subscribing to updates from relevant health authorities ensures that you receive the latest information to remain compliant with reporting requirements.
Innovations in point-of-care testing
As point-of-care testing technologies develop, integrating these results into reporting systems becomes increasingly important. Rapid tests can provide immediate results, but accurate reporting to public health authorities is essential for maintaining data integrity.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit covid-19 lab data reporting from Google Drive?
Where do I find covid-19 lab data reporting?
How do I edit covid-19 lab data reporting in Chrome?
What is covid-19 lab data reporting?
Who is required to file covid-19 lab data reporting?
How to fill out covid-19 lab data reporting?
What is the purpose of covid-19 lab data reporting?
What information must be reported on covid-19 lab data reporting?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















