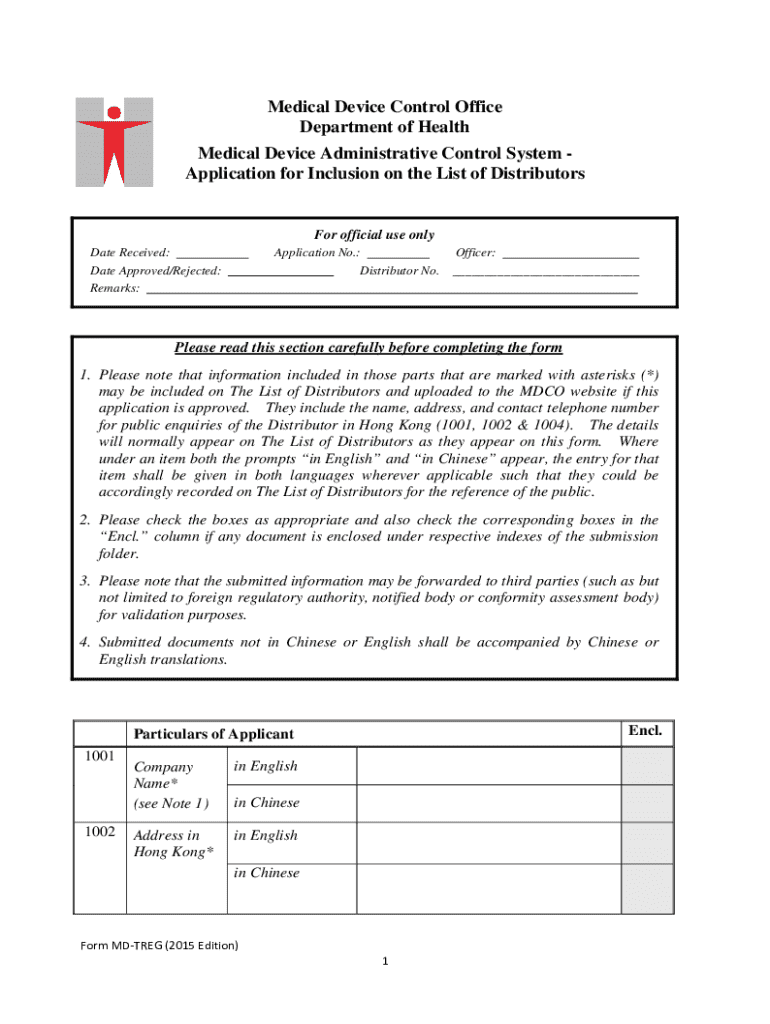
Get the free Medical Device Administrative Control System Application for Inclusion on the List o...
Get, Create, Make and Sign medical device administrative control



Editing medical device administrative control online
Uncompromising security for your PDF editing and eSignature needs
How to fill out medical device administrative control

How to fill out medical device administrative control
Who needs medical device administrative control?
Understanding the Medical Device Administrative Control Form
Overview of the medical device administrative control form
The medical device administrative control form is an essential document used in the healthcare industry to manage various aspects related to medical devices. Its primary purpose is to ensure that all necessary information related to the device is accurately captured, enabling effective tracking, regulatory compliance, and risk management. This form serves as a central repository for critical data, making it an indispensable tool in modern healthcare settings.
The importance of the medical device administrative control form cannot be overstated. It plays a crucial role in meeting regulatory requirements set forth by governing bodies like the FDA (Food and Drug Administration) and other international agencies. Compliance with these regulations guarantees that medical devices are safe, effective, and operate as intended throughout their lifecycle.
Understanding the medical device administrative control system (MDACS)
The medical device administrative control system (MDACS) is a structured framework that incorporates policies, procedures, and technologies to oversee the lifecycle of medical devices. MDACS is aimed at optimizing the management of medical devices, ensuring their efficacy, safety, and regulatory compliance throughout their use.
Key components of MDACS include surveillance mechanisms, documentation processes, and personnel training. These components work together to create a comprehensive system that not only complies with regulatory requirements but also enhances operational efficiency. By establishing robust MDACS, healthcare organizations can significantly improve the reliability of their medical device management strategies.
Key sections of the medical device administrative control form
The medical device administrative control form consists of several critical sections that facilitate the accurate capture of information about the medical device and its manufacturer. Each section serves a specific purpose that contributes to the overall integrity of the document.
Section 1: Device identification
This section requires detailed information about the medical device, including its name, model number, and unique device identifier (UDI). Providing accurate device information is crucial as it helps in effective tracking and management. Failure to list these correctly can lead to complications in compliance and device management.
Section 2: Manufacturer information
Here, essential details regarding the manufacturer must be filled out, including name, address, phone number, and contact person. This information is vital for verification purposes and enables easier communication in the case of any queries or compliance checks.
Section 3: Regulatory compliance certifications
This section requires documentation of all applicable regulatory certifications, such as FDA clearance or CE marking, signifying that the device meets safety and performance standards. Keeping this documentation updated is essential due to changes in compliance regulations.
Section 4: Product description & classification
A clear and precise product description is vital in this section, detailing how the device functions and its intended use. Accurate classification according to regulatory categories ensures compliance with specific requirements associated with each device type.
Section 5: Post-market surveillance
This section outlines the reporting requirements for any adverse events associated with the device post-approval. Best practices suggest continuous monitoring and effective reporting channels to ensure safety and efficacy throughout the product’s lifecycle.
Step-by-step guide to completing the form
Completing the medical device administrative control form can seem daunting, but a systematic approach can simplify the process. Preparation is crucial before filling out the form. Gather all necessary information and documents in advance for a smoother completion experience.
Preparation before filling out the form
Tips for information gathering include compiling device specifications, regulatory documents, and manufacturer details. Clarifying definitions and terminology related to medical devices can also ease the process of filling out the form accurately.
Detailed instructions for each section
When filling out each section, pay attention to details. Common mistakes include omitting information or listing incorrect identifiers. Double-check all entries for accuracy. Using collaborative tools such as pdfFiller can facilitate feedback and ensure all details are correctly represented.
Tips for editing and reviewing your form before submission
Before finalizing your form, it’s important to review each section thoroughly. Collaborating with colleagues can provide fresh perspectives on the information provided. Utilizing the editing features in pdfFiller enables easy adjustments and aids in creating a polished and compliant document.
Interactive tools available for the medical device administrative control form
pdfFiller offers a suite of editing tools tailored to enhance the usability of the medical device administrative control form. These features are particularly useful for teams needing to fill out, sign, and manage documents within a cloud-based environment.
pdfFiller’s editing tools overview
With user-friendly interfaces for document management, pdfFiller allows for easy modifications of existing forms, ensuring compliance standards are met. The editing tools enable users to customize the form as needed, improving accessibility and meeting specific organizational requirements.
E-signing and collaboration features
The eSigning features allow users to securely sign the medical device administrative control form digitally, eliminating the need for physical signatures and speeding up the approval process. Furthermore, collaboration functionalities enable teams to work together in real-time, making necessary adjustments and providing feedback effortlessly.
Managing your completed form
Once the medical device administrative control form is completed, managing the document effectively is crucial. Options for saving and sharing include conversion into various file formats, which can be integrated with different storage solutions to suit organizational needs.
Options for saving and sharing your document
When it comes to secure sharing practices, consider using encrypted email or secure file-sharing platforms to distribute the completed form to relevant stakeholders, ensuring that sensitive information remains protected.
Understanding retention policies for medical device forms
It's important to adhere to retention policies set forth by regulatory bodies. Typically, records related to medical devices should be retained for a predetermined period depending on the nature of the device and the applicable regulations, ensuring compliance and accountability in the management process.
Common FAQs about the medical device administrative control form
Addressing common queries about the medical device administrative control form can provide clarity for users. Many individuals wonder what to do if they’ve made a mistake on their form. Typically, it’s recommended to annotate the mistake and provide corrections rather than starting anew.
Tracking the status of submissions is also a common concern. Utilizing software tools like those from pdfFiller can help users monitor their submissions effectively, keeping them informed of the approval process.
Case studies: Effective usage of the medical device administrative control form
Real-world applications of the medical device administrative control form illustrate its efficacy in ensuring compliance and improving device management. One example includes a healthcare facility that meticulously documented compliance through proper use of the form, thereby avoiding potential regulatory fines.
Another case highlights how a team streamlined the documentation process by leveraging pdfFiller tools. This adoption resulted in improved collaboration and faster approvals, demonstrating how integrated tools can enhance workflow efficiency in medical device management.
Conclusion on the importance of accuracy and compliance
Accurate completion and management of the medical device administrative control form are critical to ensuring device safety and regulatory compliance. By systematically addressing each section of the document and leveraging the features of pdfFiller for editing and collaboration, organizations can enhance their operational efficiency and maintain compliance throughout the lifecycle of their medical devices.
The significance of this form extends beyond mere compliance; it is foundational to building a culture of safety and accountability in the medical device landscape. Therefore, healthcare organizations and professionals are encouraged to recognize the importance of this form and utilize tools provided by pdfFiller to achieve seamless document management.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit medical device administrative control from Google Drive?
How do I edit medical device administrative control online?
How do I complete medical device administrative control on an iOS device?
What is medical device administrative control?
Who is required to file medical device administrative control?
How to fill out medical device administrative control?
What is the purpose of medical device administrative control?
What information must be reported on medical device administrative control?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















